| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| UNII | DW94IAT0LS |
| EPA CompTox | DTXSID10160124 |
Structure
| InChI Key | XTKLTGBKIDQGQL-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C22H22F3N3O3 |
| Molecular Weight | 433.43 |
| AlogP | 4.26 |
| Hydrogen Bond Acceptor | 5.0 |
| Hydrogen Bond Donor | 1.0 |
| Number of Rotational Bond | 4.0 |
| Polar Surface Area | 67.59 |
| Molecular species | ACID |
| Aromatic Rings | 3.0 |
| Heavy Atoms | 31.0 |
Pharmacology
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Enzyme
Transferase
|
23-178 | 0.89-300 | - | - | - | |
|
Enzyme
|
23-178 | 0.89-300 | - | - | - |
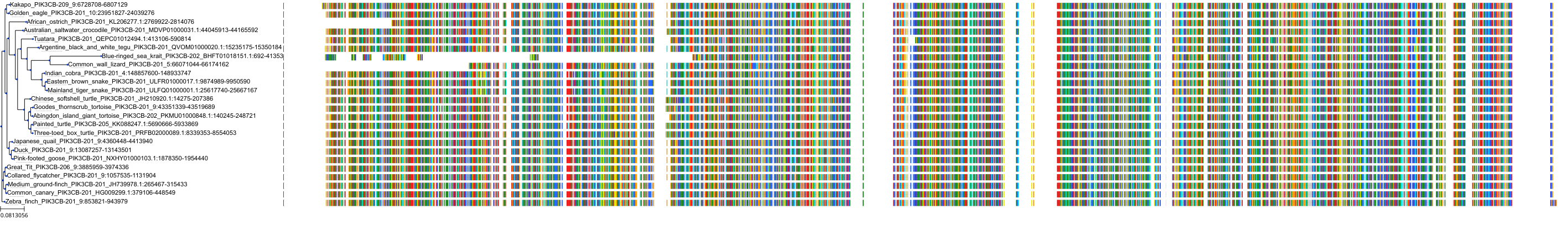
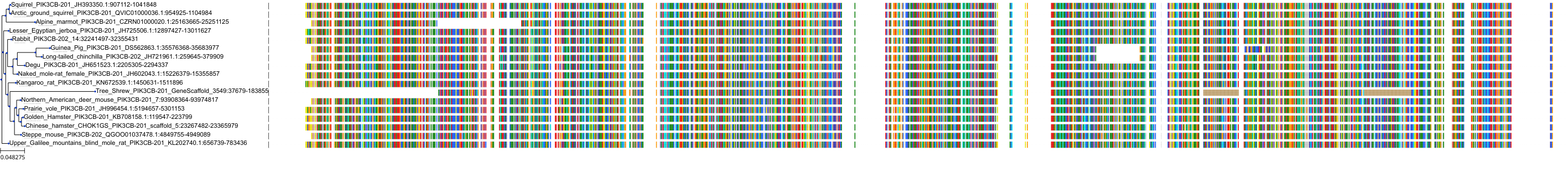
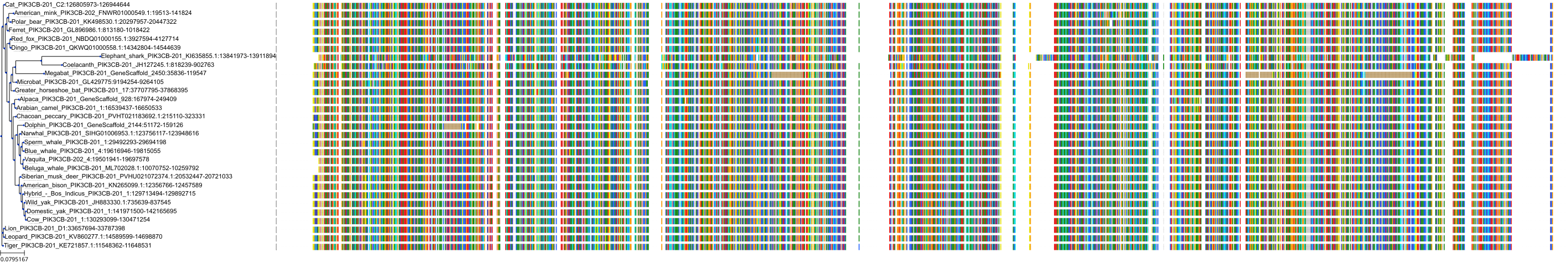
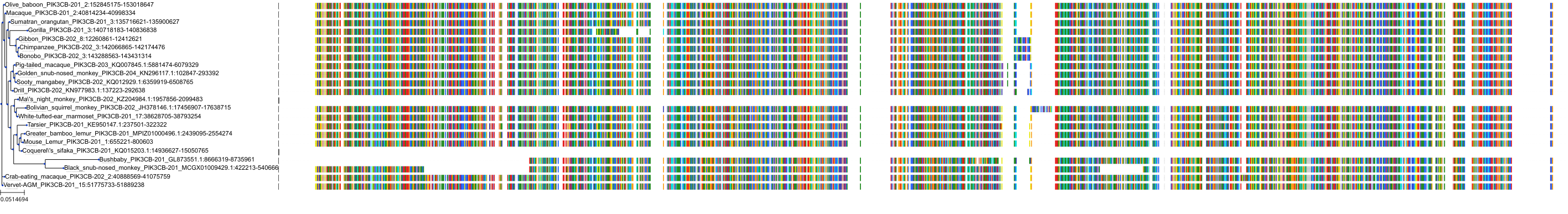
Target Conservation
|
Protein: PI3-kinase p110-beta subunit Description: Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform Organism : Homo sapiens P42338 ENSG00000051382 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL3188551 |
| DrugBank | DB11795 |
| FDA SRS | DW94IAT0LS |
| Guide to Pharmacology | 7967 |
| PubChem | 56949517 |
| SureChEMBL | SCHEMBL1280998 |
| ZINC | ZINC000077024226 |
 Homo sapiens
Homo sapiens