| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| UNII | 53S5U404NU |
| EPA CompTox | DTXSID00883395 |
Structure
| InChI Key | ANJTVLIZGCUXLD-DTWKUNHWSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C11H14N2O |
| Molecular Weight | 190.25 |
| AlogP | 0.55 |
| Hydrogen Bond Acceptor | 3.0 |
| Hydrogen Bond Donor | 1.0 |
| Polar Surface Area | 34.03 |
| Molecular species | BASE |
| Aromatic Rings | 1.0 |
| Heavy Atoms | 14.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Neuronal acetylcholine receptor; alpha4/beta2 partial agonist | PARTIAL AGONIST | PubMed PubMed |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Enzyme
Hydrolase
|
- | - | - | - | -3.85-2.17 | |
|
Ion channel
Ligand-gated ion channel
Nicotinic acetylcholine receptor
Nicotinic acetylcholine receptor alpha subunit
|
0.42-25 | 50-69.18 | - | 0.122-840 | 30-30 | |
|
Ion channel
Ligand-gated ion channel
Nicotinic acetylcholine receptor
Nicotinic acetylcholine receptor beta subunit
|
0.42-25 | 50-69.18 | - | 0.122-840 | 30-30 | |
|
Ion channel
Ligand-gated ion channel
Nicotinic acetylcholine receptor
Nicotinic acetylcholine receptor delta subunit
|
11 | - | - | 250-250 | - | |
|
Ion channel
Ligand-gated ion channel
Nicotinic acetylcholine receptor
Nicotinic acetylcholine receptor gamma subunit
|
11 | - | - | 250-250 | - | |
|
Transporter
Electrochemical transporter
SLC superfamily of solute carriers
SLC21/SLCO family of organic anion transporting polypeptides
|
- | - | - | - | 99.71-106.19 |
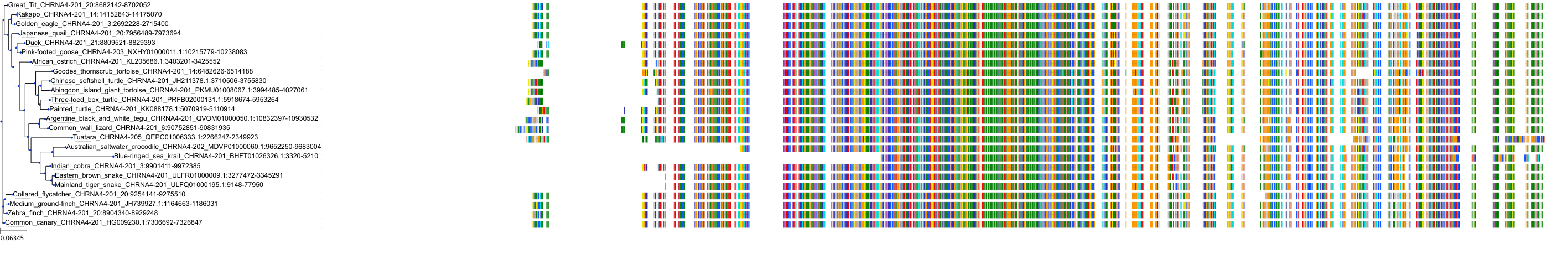
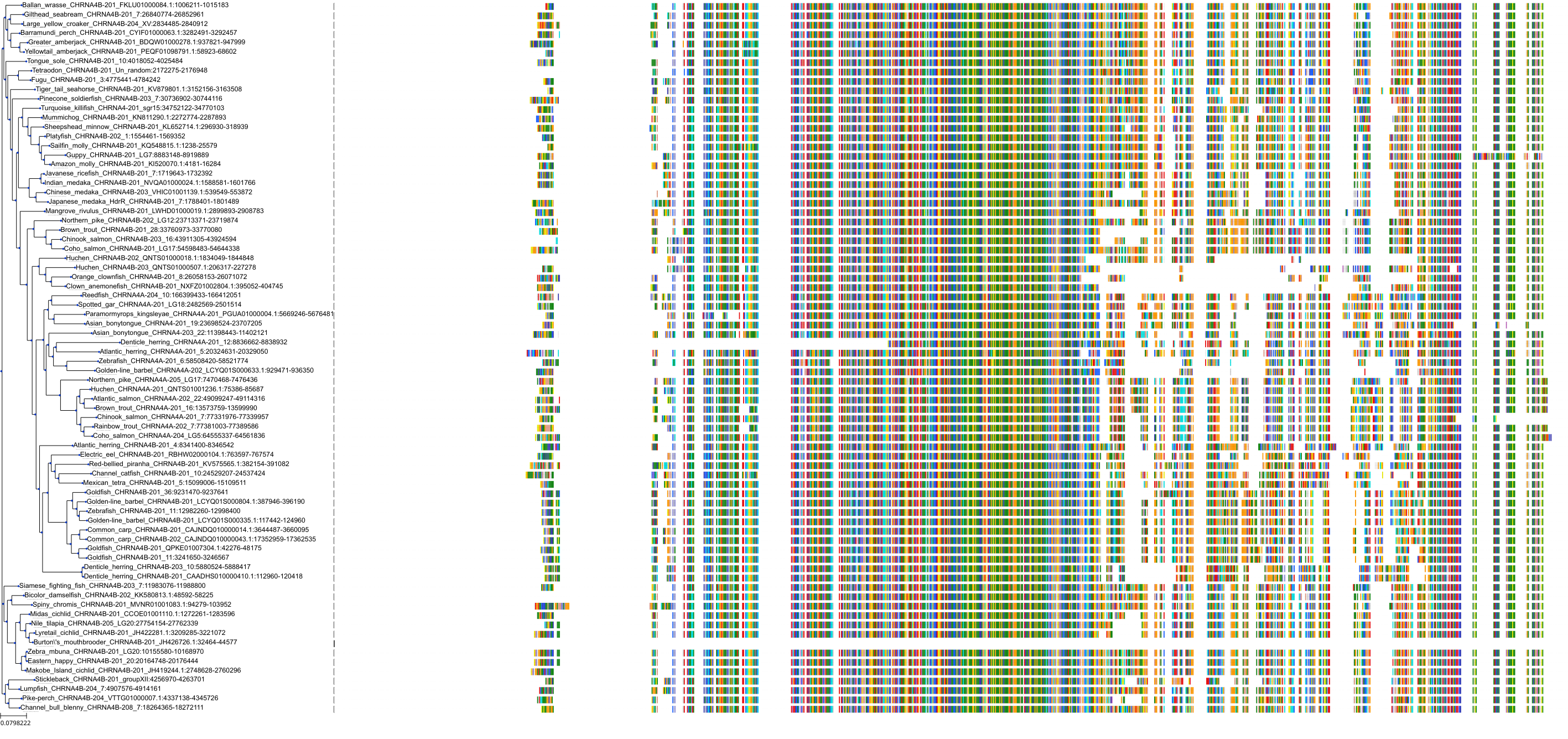
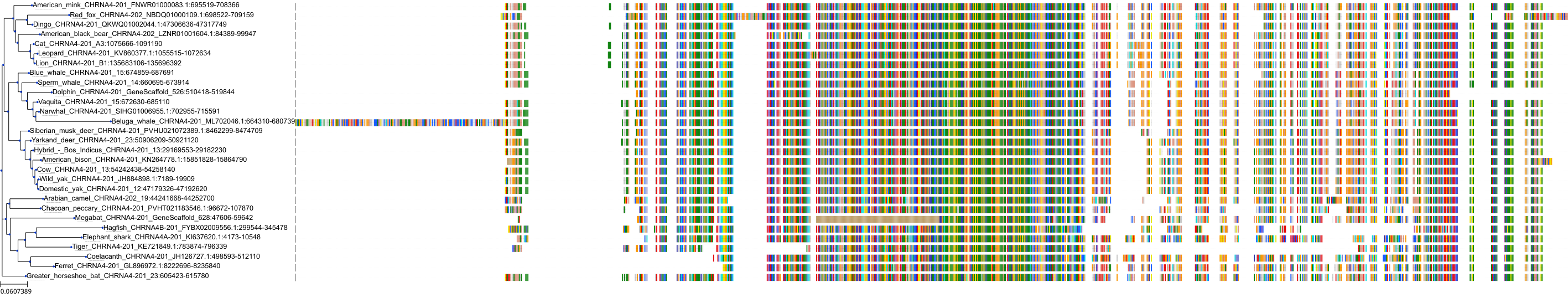
Target Conservation
|
Protein: Neuronal acetylcholine receptor; alpha4/beta2 Description: Neuronal acetylcholine receptor subunit beta-2 Organism : Homo sapiens P17787 ENSG00000160716 |
||||
|
Protein: Neuronal acetylcholine receptor; alpha4/beta2 Description: Neuronal acetylcholine receptor subunit alpha-4 Organism : Homo sapiens P43681 ENSG00000101204 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 4055 |
| ChEMBL | CHEMBL497939 |
| DrugBank | DB09028 |
| DrugCentral | 5217 |
| FDA SRS | 53S5U404NU |
| Guide to Pharmacology | 5347 |
| PDB | C5E |
| PubChem | 10235 |
| SureChEMBL | SCHEMBL161398 |
| ZINC | ZINC000001599730 |
 Apis mellifera
Apis mellifera
 Bos taurus
Bos taurus
 Cricetulus griseus
Cricetulus griseus
 Electrophorus electricus
Electrophorus electricus
 Equus caballus
Equus caballus
 Homo sapiens
Homo sapiens
 Rattus norvegicus
Rattus norvegicus
 Sus scrofa
Sus scrofa
 pisces
pisces