Structure
| InChI Key | RPCVIAXDAUMJJP-PZBABLGHSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C14H22N2O |
| Molecular Weight | 234.34 |
| AlogP | 2.88 |
| Hydrogen Bond Acceptor | 3.0 |
| Hydrogen Bond Donor | 1.0 |
| Number of Rotational Bond | 6.0 |
| Polar Surface Area | 34.15 |
| Molecular species | BASE |
| Aromatic Rings | 1.0 |
| Heavy Atoms | 17.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Neuronal acetylcholine receptor; alpha4/beta2 partial agonist | PARTIAL AGONIST | Other Wikipedia PubMed |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Ion channel
Ligand-gated ion channel
Nicotinic acetylcholine receptor
Nicotinic acetylcholine receptor alpha subunit
|
106-220 | - | - | - | - | |
|
Ion channel
Ligand-gated ion channel
Nicotinic acetylcholine receptor
Nicotinic acetylcholine receptor beta subunit
|
106-220 | - | - | - | - |
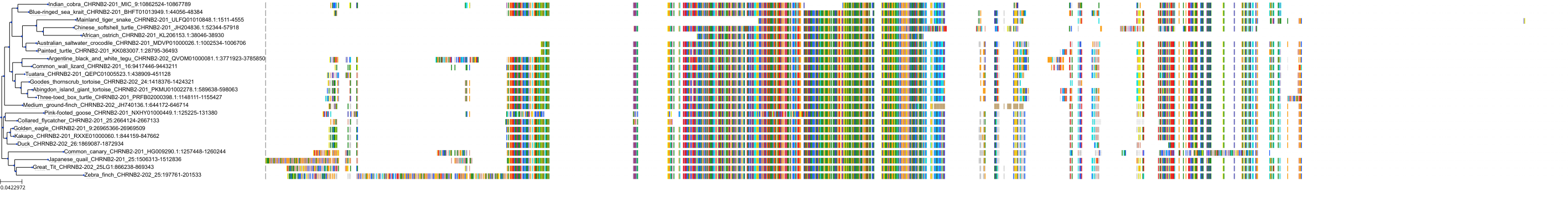
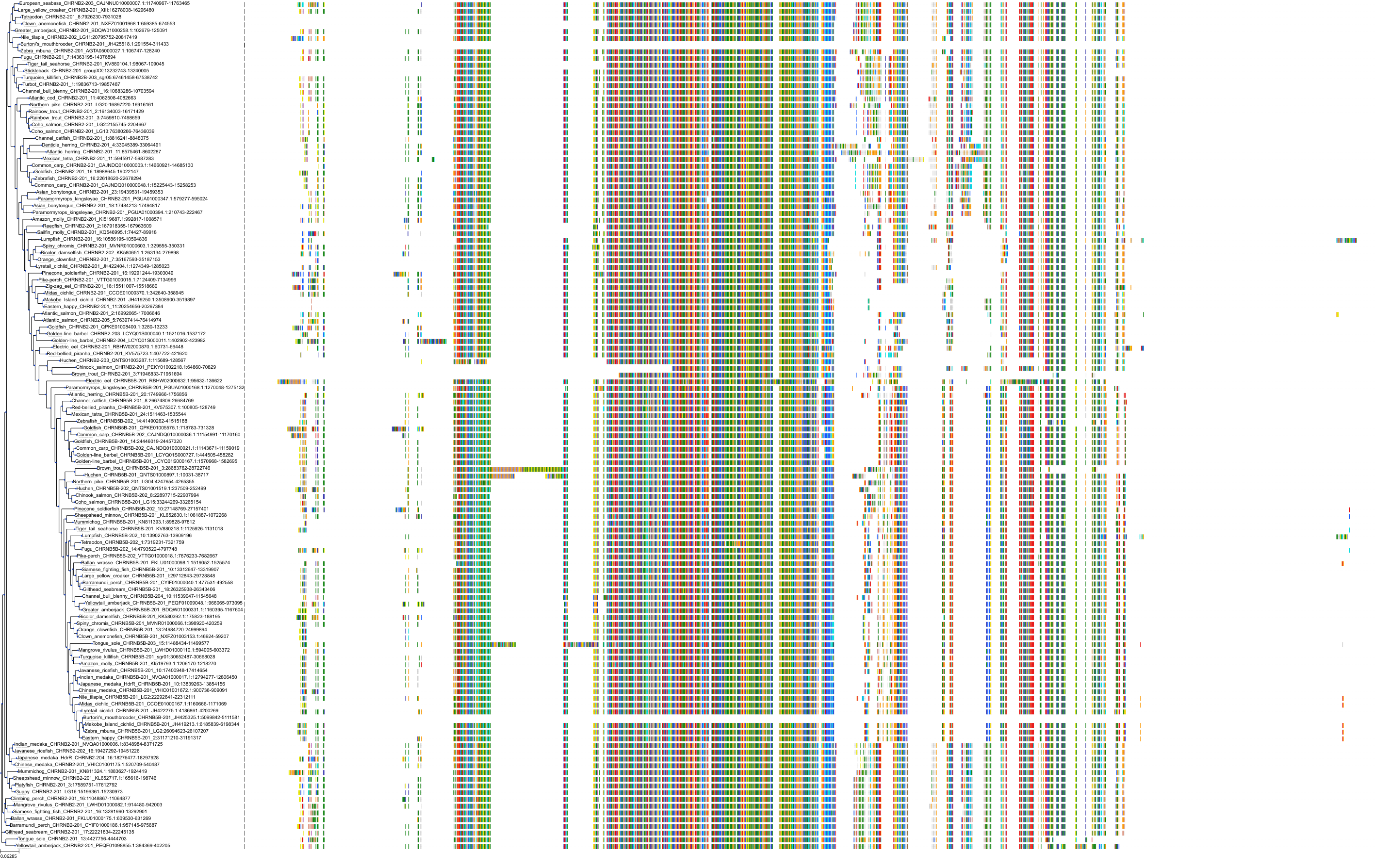
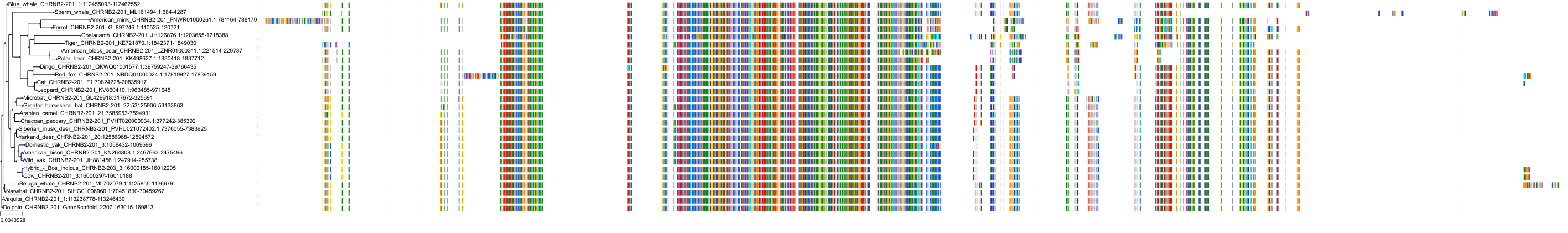
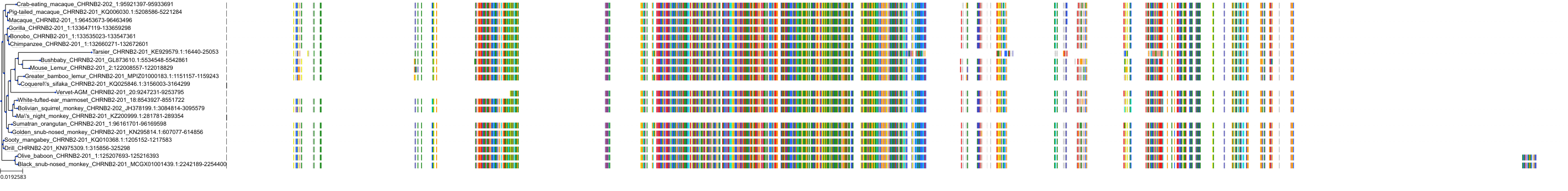
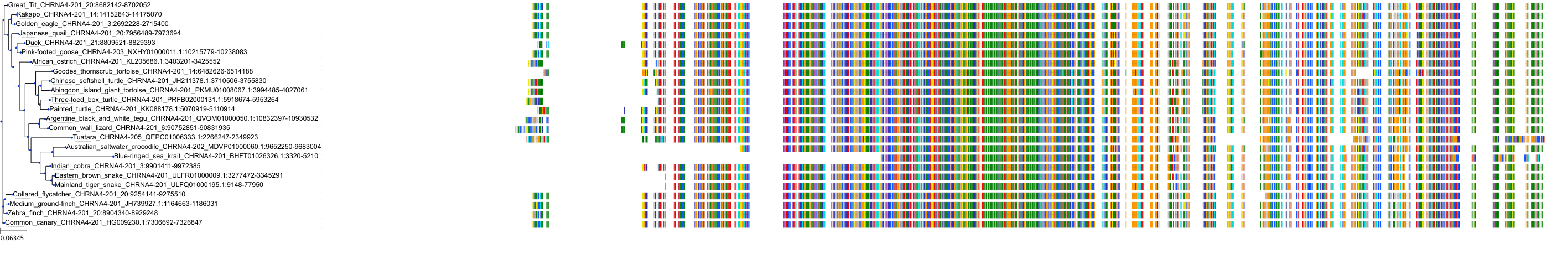
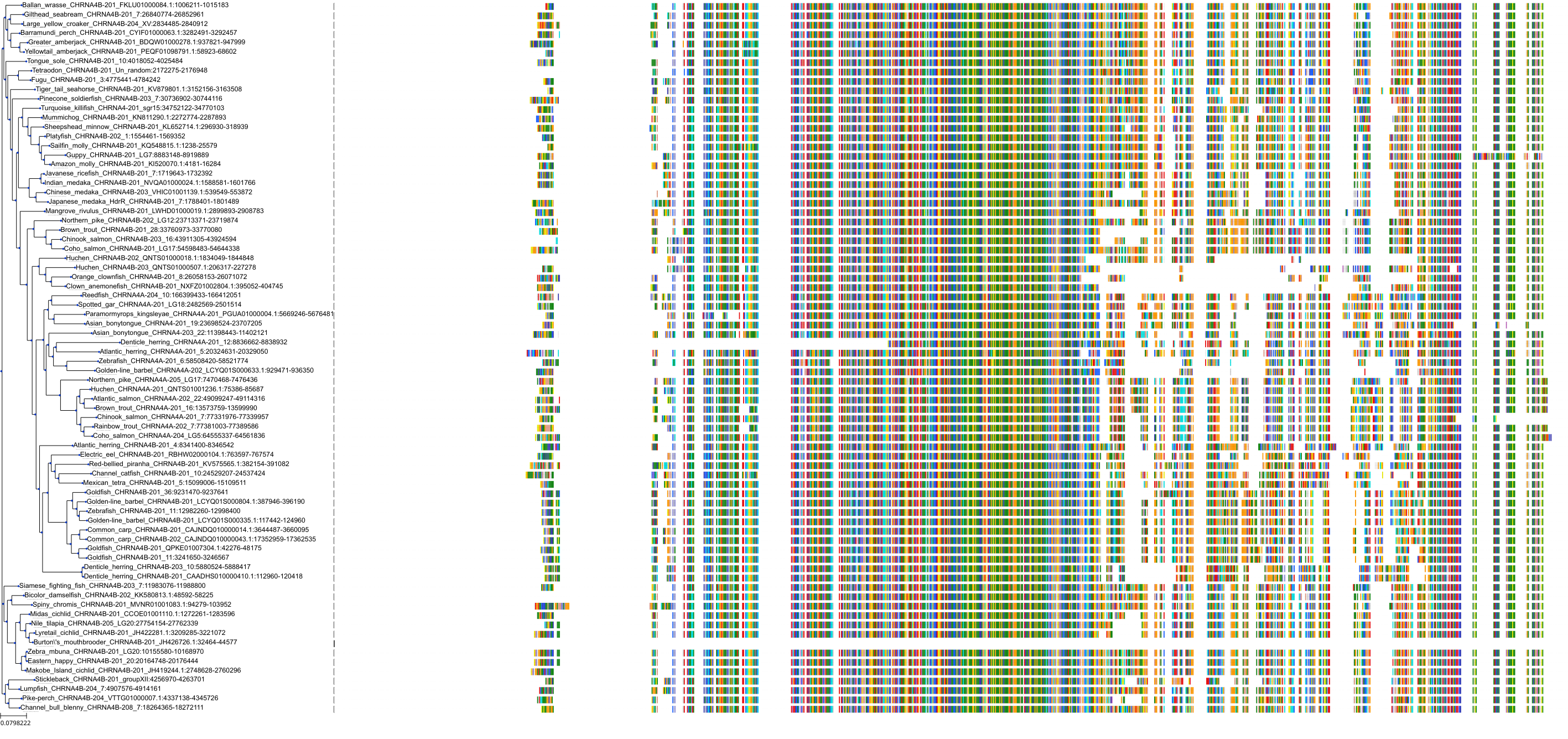
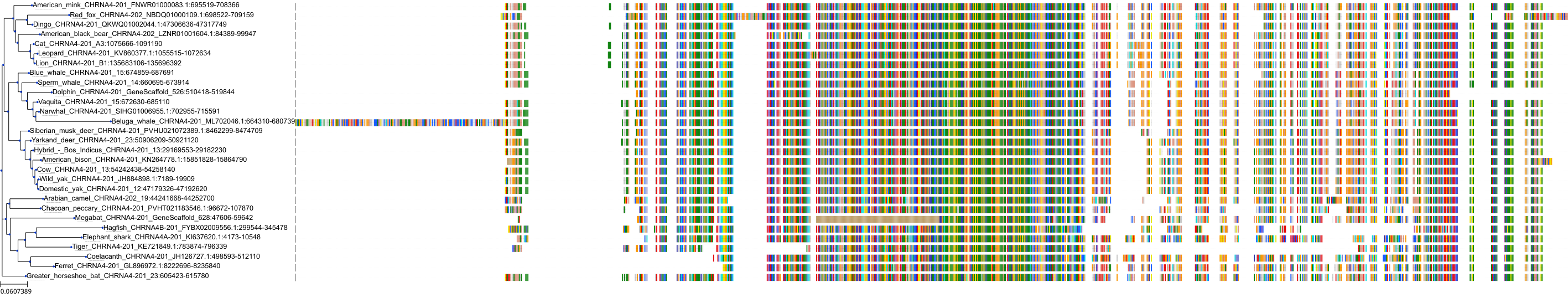
Target Conservation
|
Protein: Neuronal acetylcholine receptor; alpha4/beta2 Description: Neuronal acetylcholine receptor subunit beta-2 Organism : Homo sapiens P17787 ENSG00000160716 |
||||
|
Protein: Neuronal acetylcholine receptor; alpha4/beta2 Description: Neuronal acetylcholine receptor subunit alpha-4 Organism : Homo sapiens P43681 ENSG00000101204 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL188462 |
| DrugBank | DB16205 |
| FDA SRS | 3E05NBH9V5 |
| PubChem | 9824145 |
| SureChEMBL | SCHEMBL366585 |
| ZINC | ZINC000003961864 |
 Homo sapiens
Homo sapiens
 Rattus norvegicus
Rattus norvegicus