Structure
| InChI Key | JYTIXGYXBIBOMN-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C27H22ClN3O4 |
| Molecular Weight | 487.94 |
| AlogP | 5.17 |
| Hydrogen Bond Acceptor | 5.0 |
| Hydrogen Bond Donor | 1.0 |
| Number of Rotational Bond | 6.0 |
| Polar Surface Area | 84.66 |
| Molecular species | ACID |
| Aromatic Rings | 4.0 |
| Heavy Atoms | 35.0 |
Pharmacology
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Transcription factor
Nuclear receptor
Nuclear hormone receptor subfamily 1
Nuclear hormone receptor subfamily 1 group H
Nuclear hormone receptor subfamily 1 group H member 4
|
7-32 | - | - | - | - |
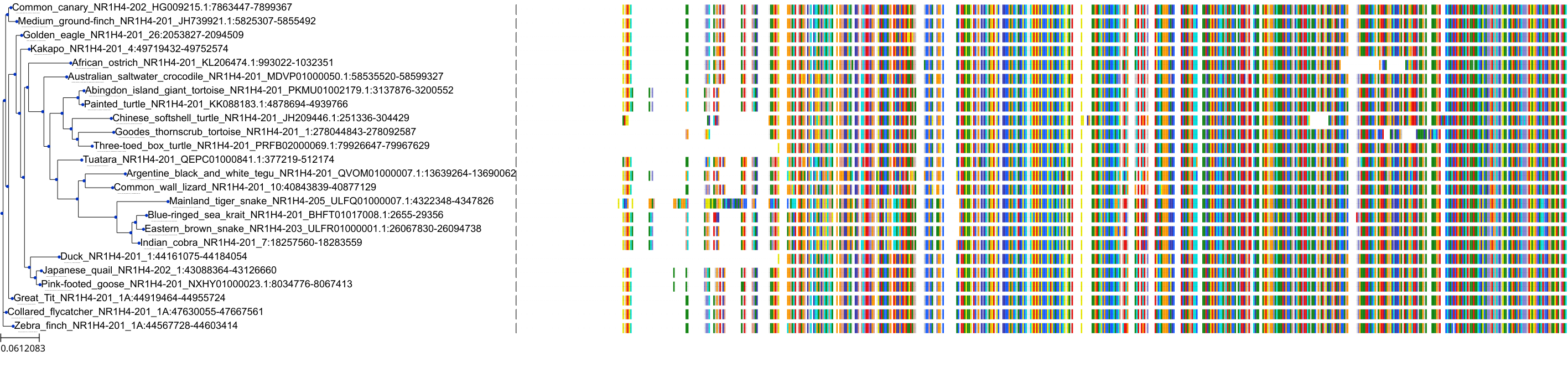
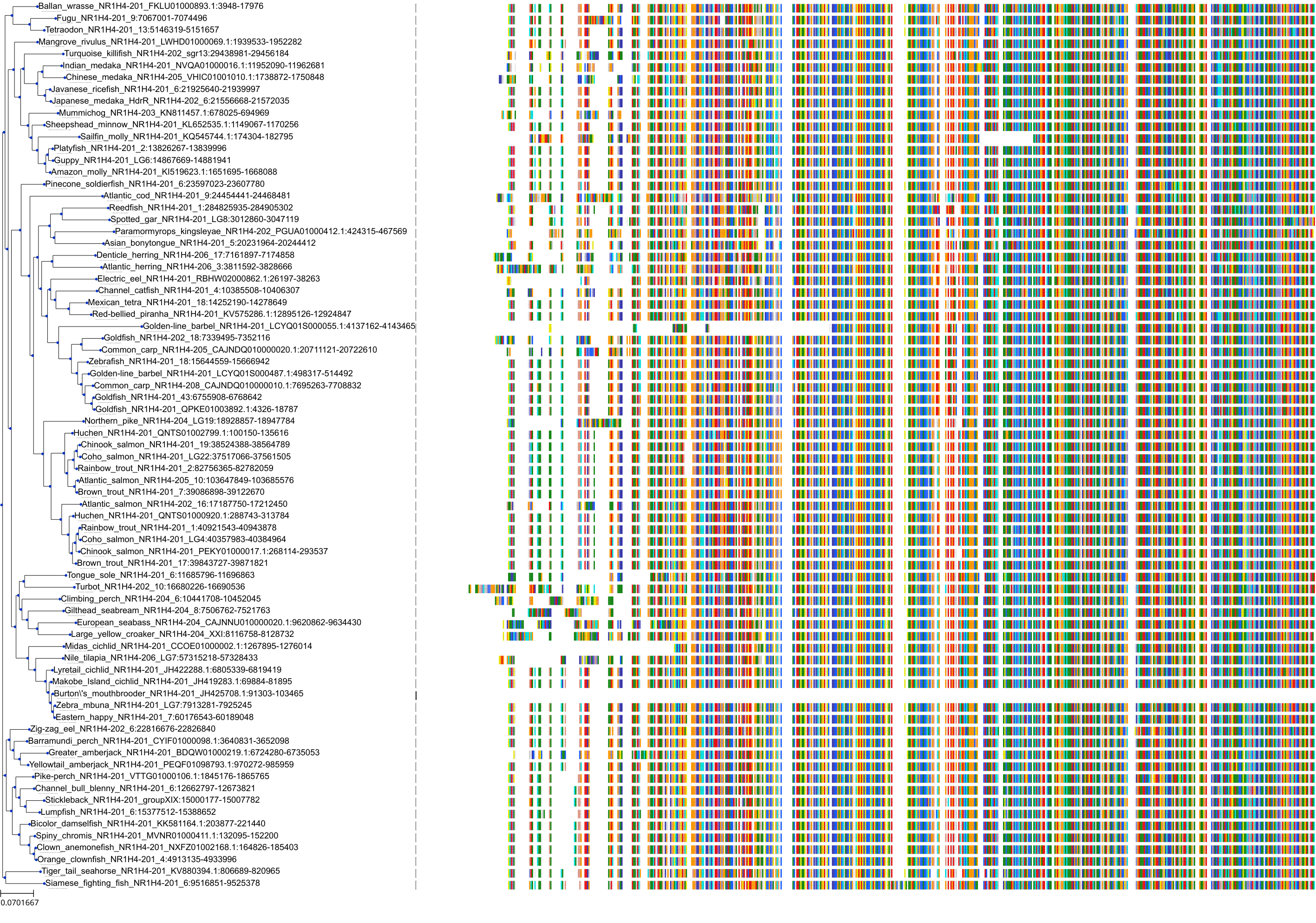
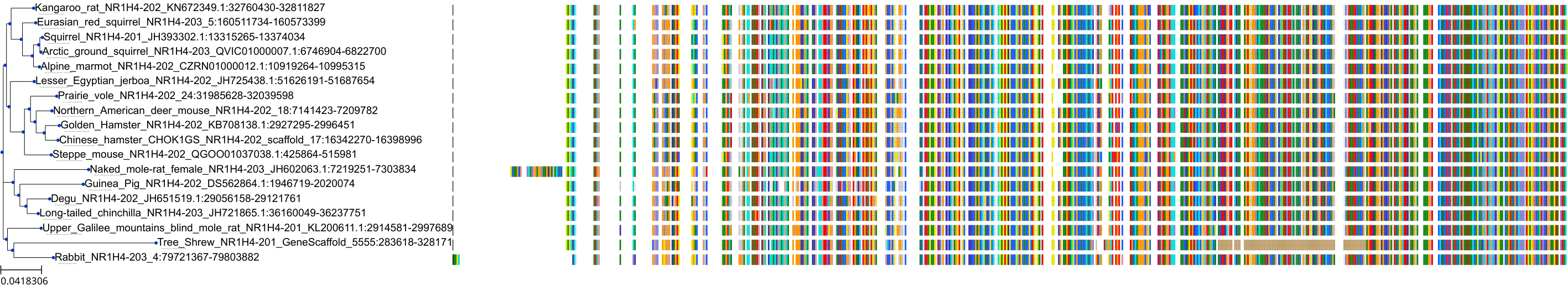
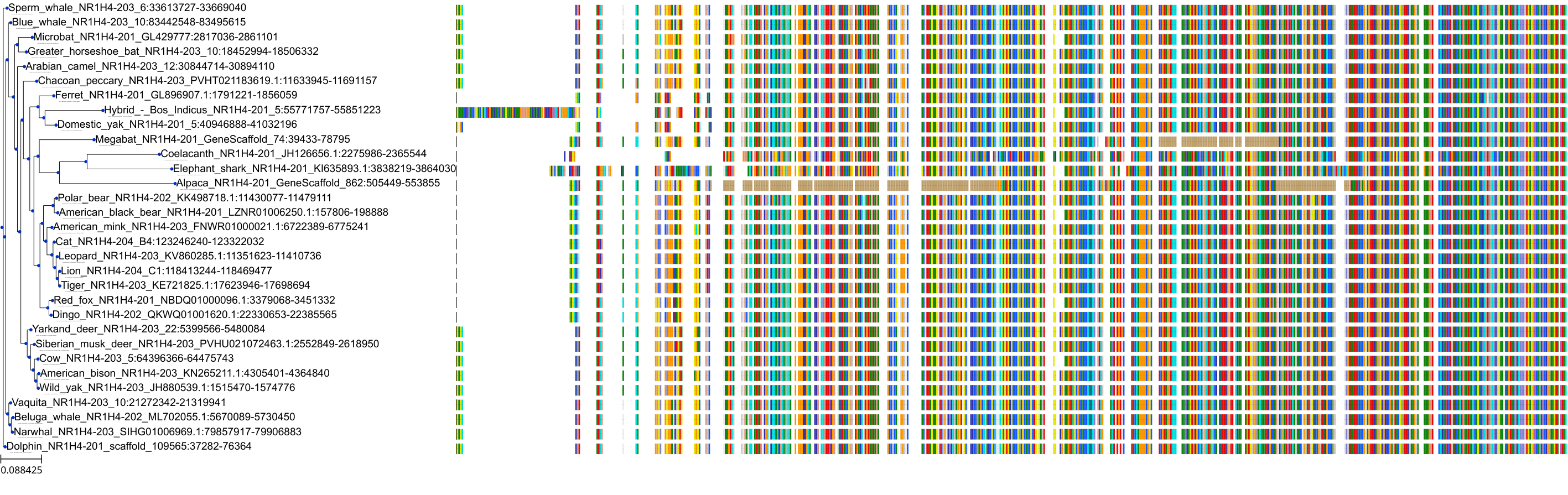
Target Conservation
|
Protein: Bile acid receptor FXR Description: Bile acid receptor Organism : Homo sapiens Q96RI1 ENSG00000012504 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL4297626 |
| DrugBank | DB16255 |
| FDA SRS | CJ1PL0TE6J |
| Guide to Pharmacology | 10655 |
| PubChem | 118063735 |
| SureChEMBL | SCHEMBL16702097 |
| ZINC | ZINC000584641402 |
 Homo sapiens
Homo sapiens