| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| UNII | Y1NA96IX3T |
| EPA CompTox | DTXSID20239496 |
Structure
| InChI Key | DFQGDHBGRSTTHX-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C38H43N3O4S |
| Molecular Weight | 637.85 |
| AlogP | 8.98 |
| Hydrogen Bond Acceptor | 7.0 |
| Hydrogen Bond Donor | 1.0 |
| Number of Rotational Bond | 12.0 |
| Polar Surface Area | 86.47 |
| Molecular species | ACID |
| Aromatic Rings | 5.0 |
| Heavy Atoms | 46.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| 5-lipoxygenase activating protein inhibitor | INHIBITOR | PubMed PubMed |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Other cytosolic protein
|
- | 0.7-506 | - | - | - |
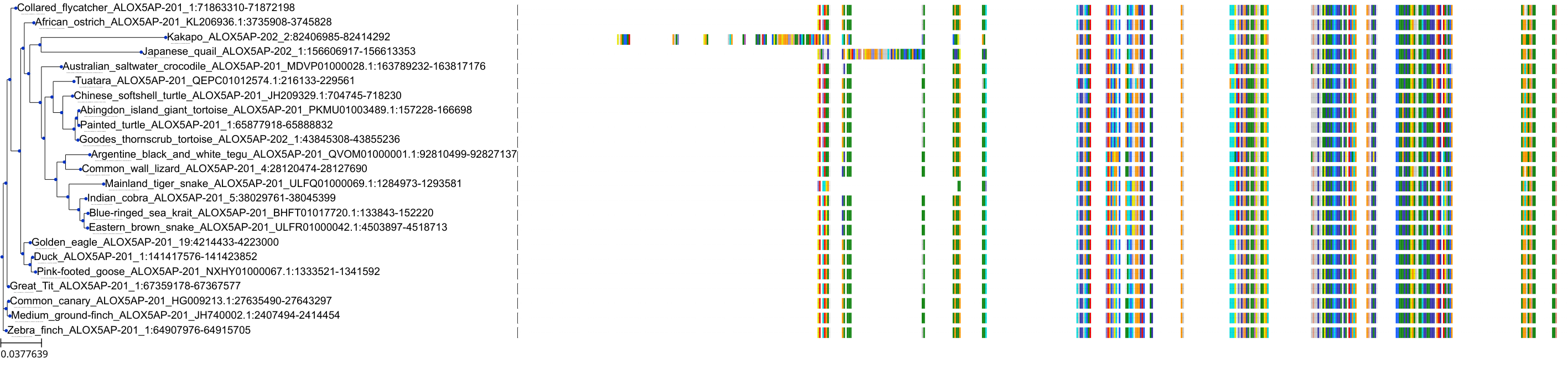
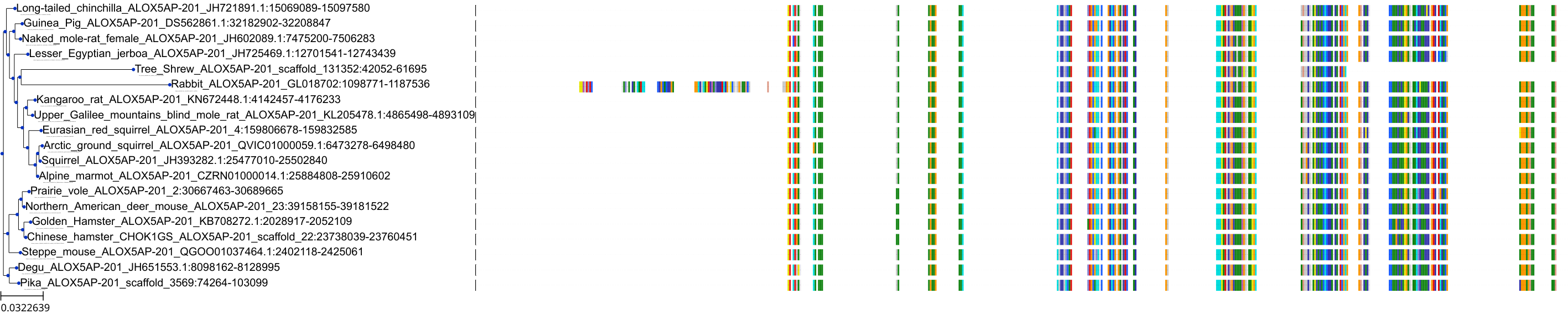
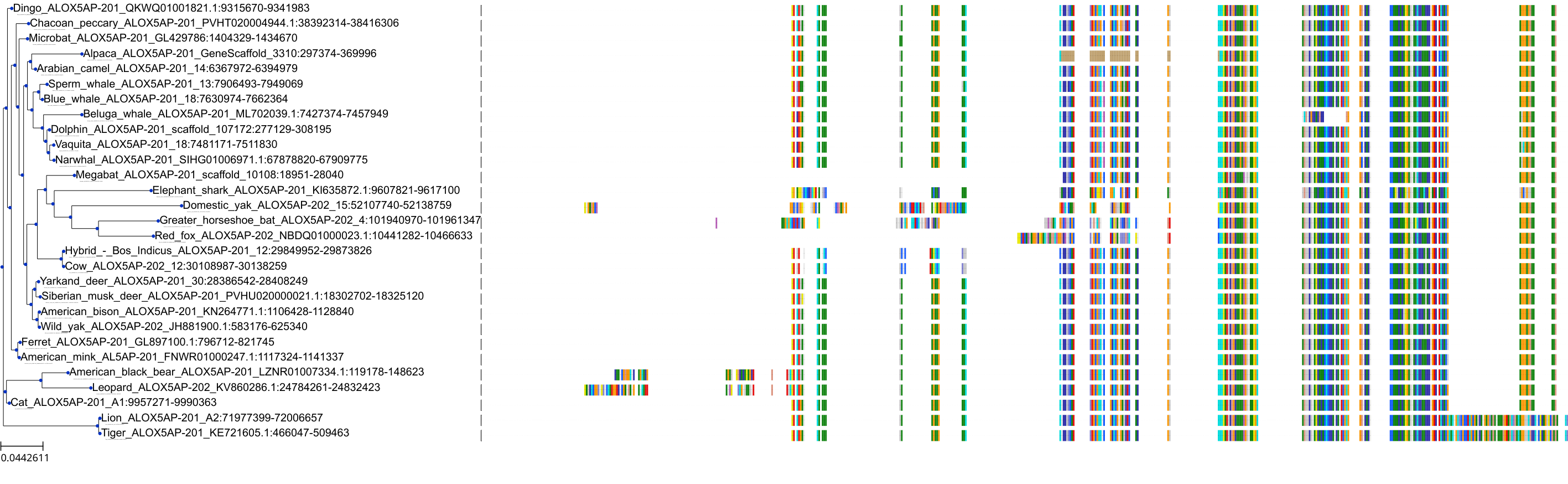
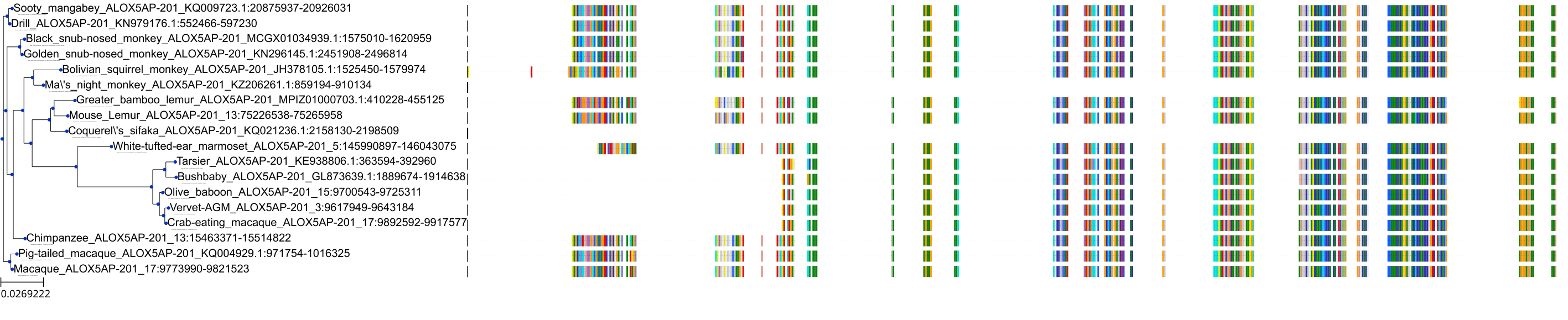
Target Conservation
|
Protein: 5-lipoxygenase activating protein Description: Arachidonate 5-lipoxygenase-activating protein Organism : Homo sapiens P20292 ENSG00000132965 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL1922660 |
| DrugBank | DB06346 |
| FDA SRS | Y1NA96IX3T |
| PubChem | 44473151 |
| SureChEMBL | SCHEMBL11820 |
| ZINC | ZINC000068247071 |
 Homo sapiens
Homo sapiens
 Rattus norvegicus
Rattus norvegicus