Structure
| InChI Key | TZSZZENYCISATO-WIOPSUGQSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C29H31ClF3N5O3 |
| Molecular Weight | 590.05 |
| AlogP | 5.57 |
| Hydrogen Bond Acceptor | 8.0 |
| Hydrogen Bond Donor | 2.0 |
| Number of Rotational Bond | 7.0 |
| Polar Surface Area | 102.6 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 3.0 |
| Heavy Atoms | 41.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Tryptophan 5-hydroxylase 1 inhibitor | INHIBITOR | Other |
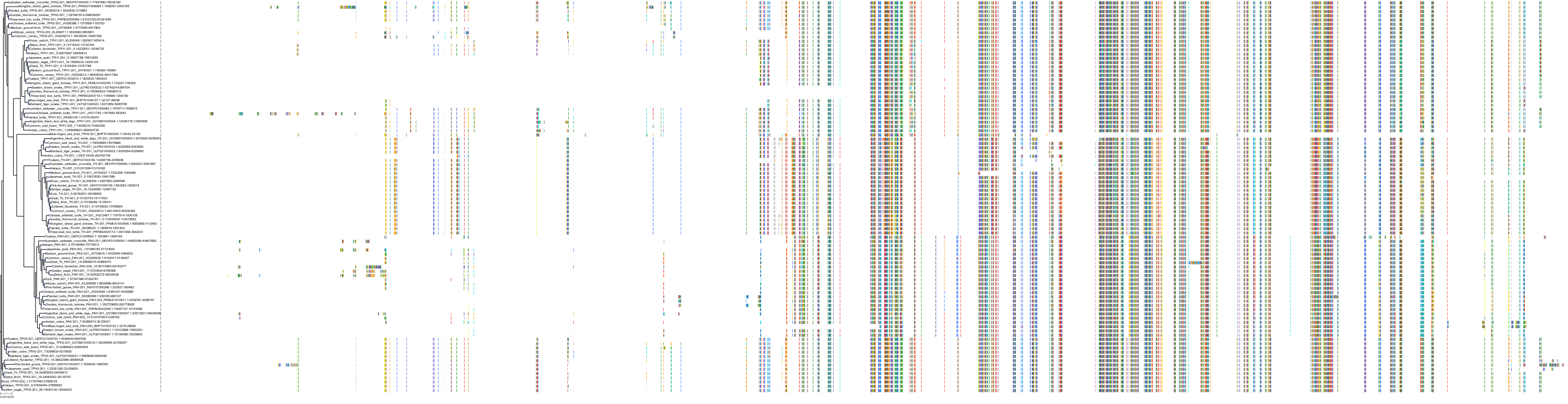
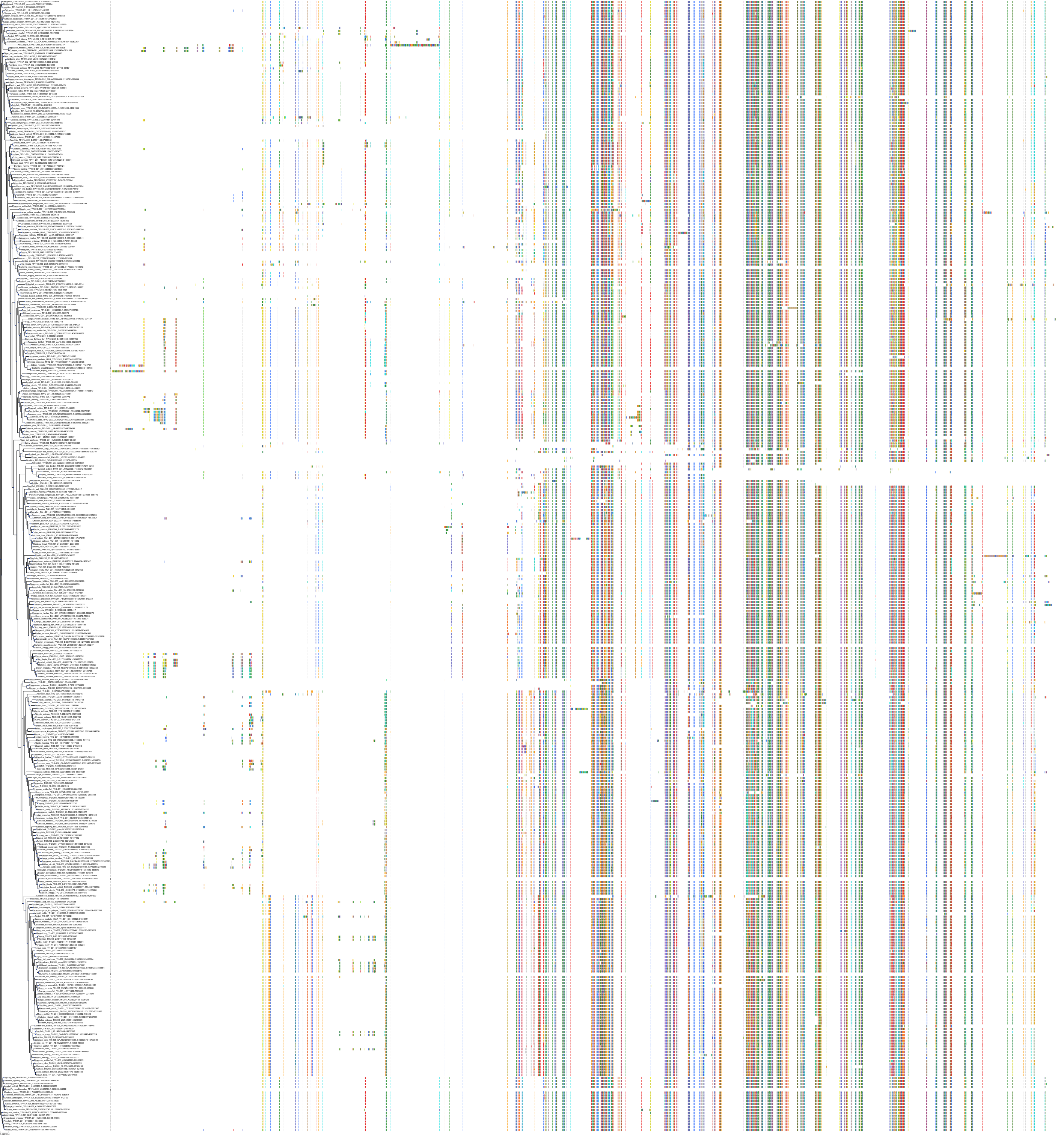
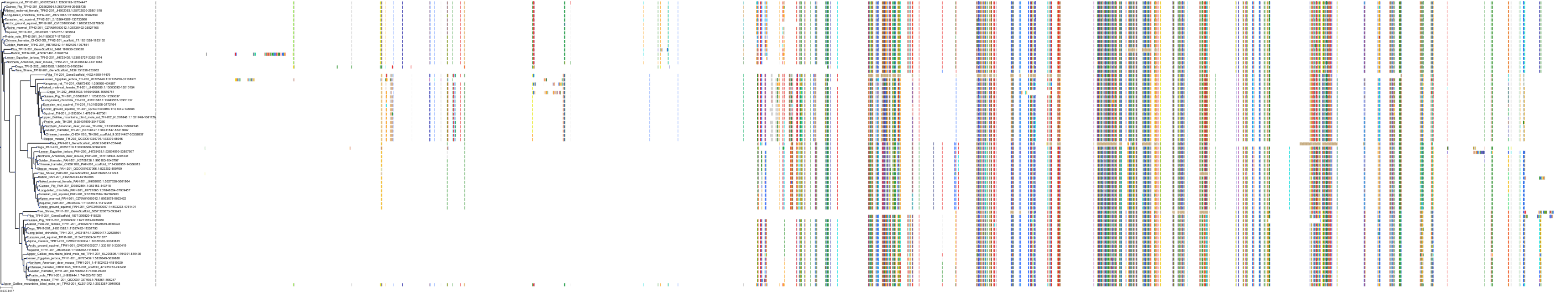
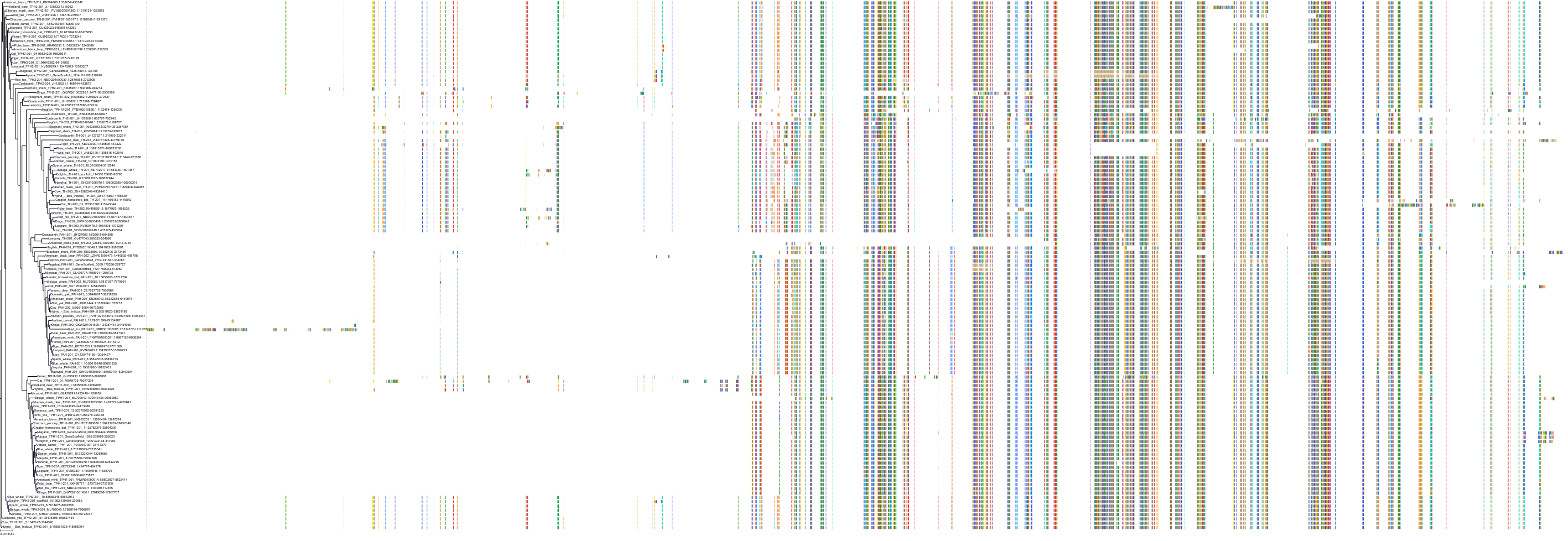
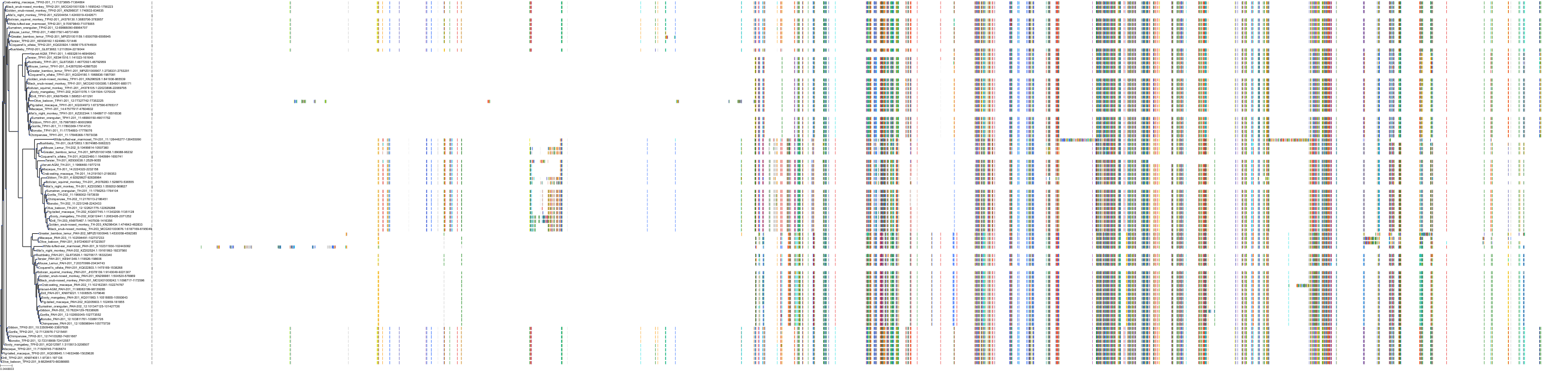
Target Conservation
|
Protein: Tryptophan 5-hydroxylase 1 Description: Tryptophan 5-hydroxylase 1 Organism : Homo sapiens P17752 ENSG00000129167 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL4069704 |
| FDA SRS | 507FY6OL37 |
| Guide to Pharmacology | 10646 |
| PubChem | 92045025 |
| SureChEMBL | SCHEMBL16573975 |
 Mus musculus
Mus musculus