| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| UNII | Z07JR07J6C |
| EPA CompTox | DTXSID80167504 |
Structure
| InChI Key | IPSYPUKKXMNCNQ-PFHKOEEOSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C18H18ClIN6O4 |
| Molecular Weight | 544.74 |
| AlogP | 1.06 |
| Hydrogen Bond Acceptor | 9.0 |
| Hydrogen Bond Donor | 4.0 |
| Number of Rotational Bond | 5.0 |
| Polar Surface Area | 134.42 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 3.0 |
| Heavy Atoms | 30.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Adenosine A3 receptor agonist | AGONIST | PubMed |
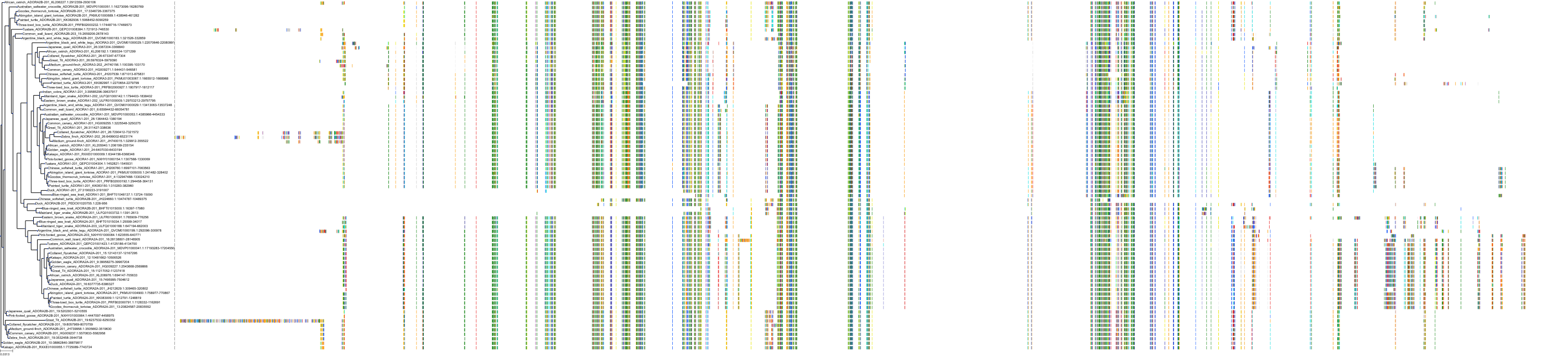
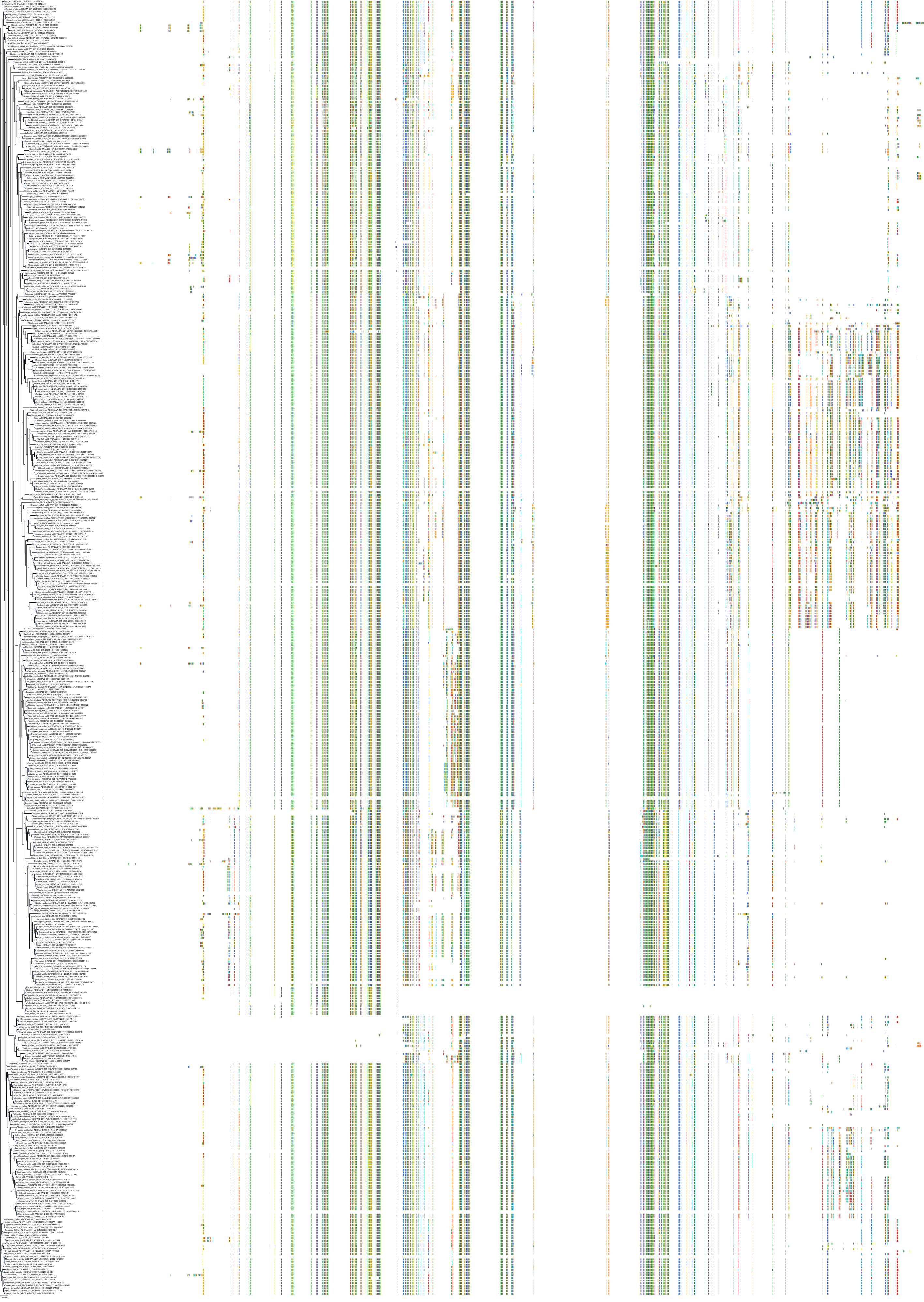
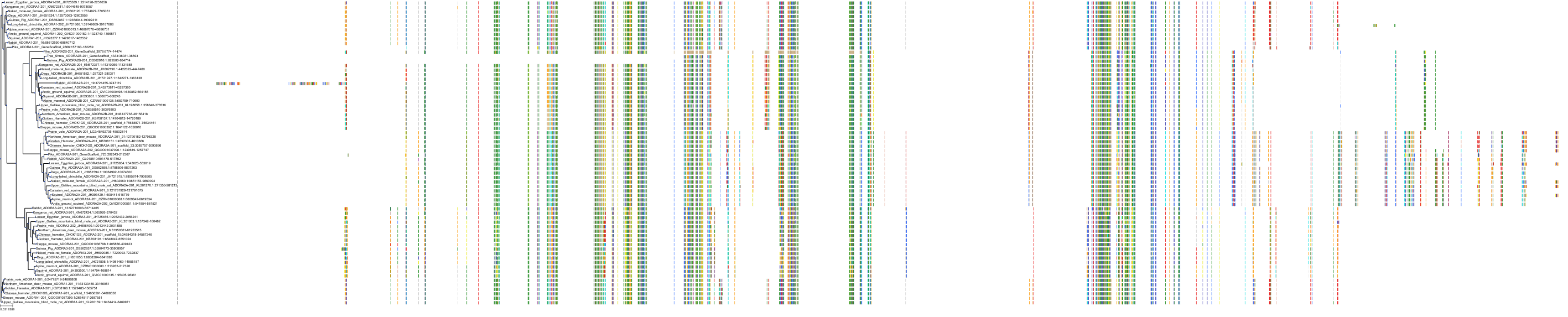
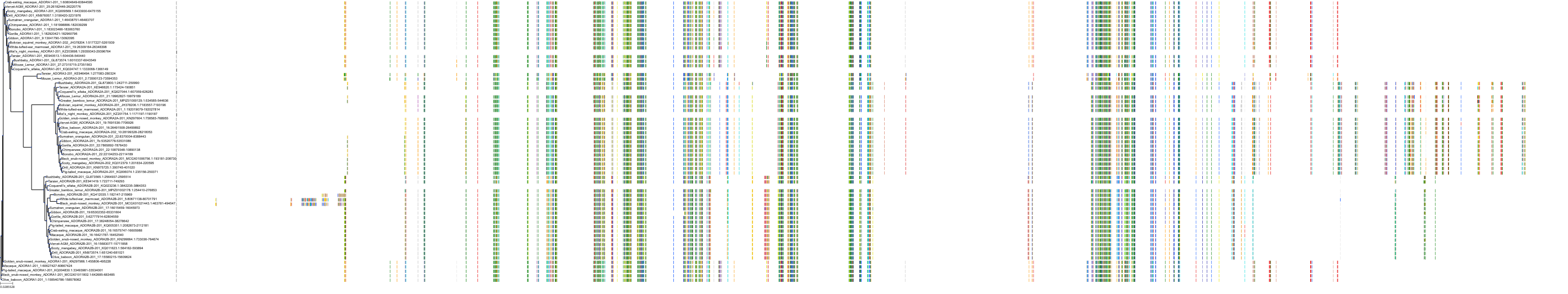
Target Conservation
|
Protein: Adenosine A3 receptor Description: Adenosine receptor A3 Organism : Homo sapiens P0DMS8 ENSG00000282608 |
||||
Related Entries
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL431733 |
| DrugBank | DB12885 |
| FDA SRS | Z07JR07J6C |
| Guide to Pharmacology | 457 |
| PubChem | 3035850 |
| SureChEMBL | SCHEMBL1170028 |
| ZINC | ZINC000003995845 |
 Bos taurus
Bos taurus
 Homo sapiens
Homo sapiens
 Mus musculus
Mus musculus
 Rattus norvegicus
Rattus norvegicus