| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| UNII | K5BX8ZA7UF |
| EPA CompTox | DTXSID10471000 |
Structure
| InChI Key | MZDKLVOWGIOKTN-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C21H20F3N7O3S |
| Molecular Weight | 507.5 |
| AlogP | 3.14 |
| Hydrogen Bond Acceptor | 8.0 |
| Hydrogen Bond Donor | 3.0 |
| Number of Rotational Bond | 7.0 |
| Polar Surface Area | 129.21 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 3.0 |
| Heavy Atoms | 35.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Focal adhesion kinase 1 inhibitor | INHIBITOR | PubMed |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Enzyme
Cytochrome P450
Cytochrome P450 family 1
Cytochrome P450 family 1A
Cytochrome P450 1A2
|
- | - | - | - | 91.2 | |
|
Enzyme
Cytochrome P450
Cytochrome P450 family 2
Cytochrome P450 family 2C
Cytochrome P450 2C19
|
- | - | - | - | 843 | |
|
Enzyme
Cytochrome P450
Cytochrome P450 family 2
Cytochrome P450 family 2C
Cytochrome P450 2C9
|
- | - | - | - | 42.7 | |
|
Enzyme
Cytochrome P450
Cytochrome P450 family 2
Cytochrome P450 family 2D
Cytochrome P450 2D6
|
- | - | - | - | 90.5 | |
|
Enzyme
Cytochrome P450
Cytochrome P450 family 3
Cytochrome P450 family 3A
Cytochrome P450 3A4
|
- | - | - | - | 47.2 | |
|
Enzyme
Kinase
Protein Kinase
TK protein kinase group
Tyrosine protein kinase Fak family
|
- | 1-14 | - | - | - |
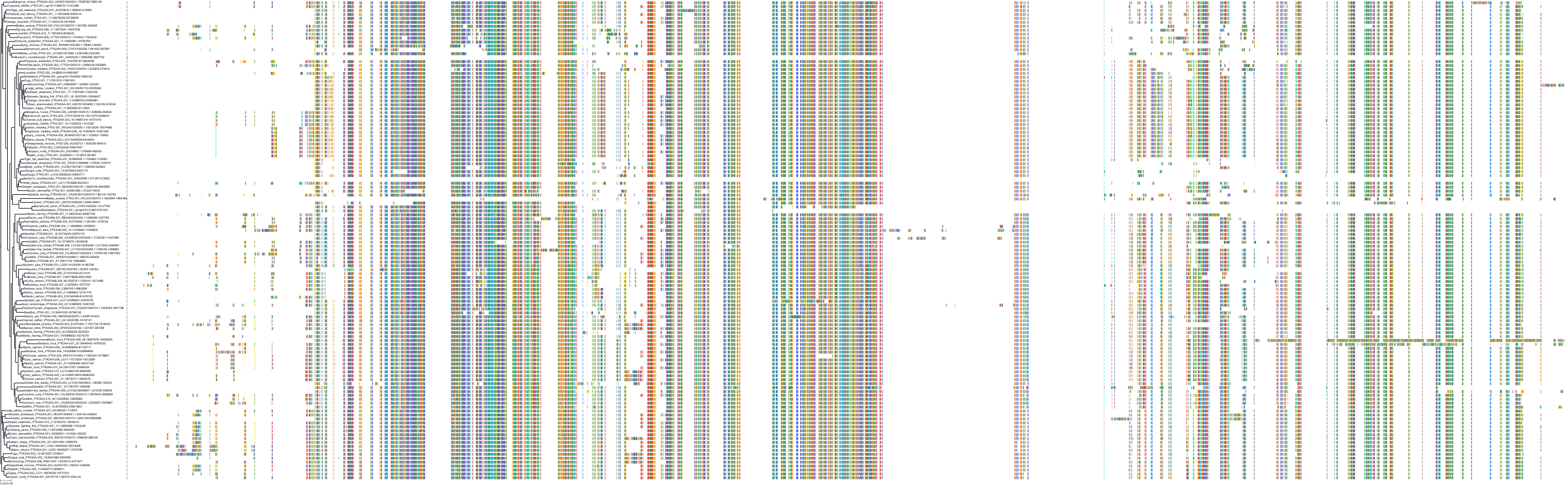
Target Conservation
|
Protein: Focal adhesion kinase 1 Description: Focal adhesion kinase 1 Organism : Homo sapiens Q05397 ENSG00000169398 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 91370 |
| ChEMBL | CHEMBL1084546 |
| FDA SRS | K5BX8ZA7UF |
| Guide to Pharmacology | 9381 |
| PDB | YAM |
| PubChem | 16118986 |
| SureChEMBL | SCHEMBL1206959 |
| ZINC | ZINC000034638188 |
 Homo sapiens
Homo sapiens