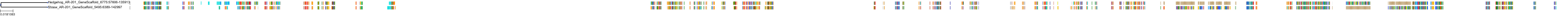| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| ATC | L02BX03 |
| UNII | G819A456D0 |
| EPA CompTox | DTXSID80879993 |
Structure
| InChI Key | GZOSMCIZMLWJML-VJLLXTKPSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C24H31NO |
| Molecular Weight | 349.52 |
| AlogP | 5.4 |
| Hydrogen Bond Acceptor | 2.0 |
| Hydrogen Bond Donor | 1.0 |
| Number of Rotational Bond | 1.0 |
| Polar Surface Area | 33.12 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 1.0 |
| Heavy Atoms | 26.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Androgen Receptor antagonist | ANTAGONIST | PubMed PubMed PubMed PubMed PubMed |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Enzyme
Cytochrome P450
Cytochrome P450 family 1
Cytochrome P450 family 1A
Cytochrome P450 1A2
|
- | - | - | - | 34-92.5 | |
|
Enzyme
Cytochrome P450
Cytochrome P450 family 17
Cytochrome P450 family 17A
Cytochrome P450 17A1
|
- | 2.9-800 | - | - | 74-100 | |
|
Enzyme
Cytochrome P450
Cytochrome P450 family 2
Cytochrome P450 family 2B
Cytochrome P450 2B6
|
- | - | - | - | 2-11 | |
|
Enzyme
Cytochrome P450
Cytochrome P450 family 2
Cytochrome P450 family 2C
Cytochrome P450 2C19
|
- | - | - | - | 3-91.6 | |
|
Enzyme
Cytochrome P450
Cytochrome P450 family 2
Cytochrome P450 family 2C
Cytochrome P450 2C9
|
- | - | - | - | 17-82.5 | |
|
Enzyme
Cytochrome P450
Cytochrome P450 family 2
Cytochrome P450 family 2D
Cytochrome P450 2D6
|
- | - | - | - | 7-78.5 | |
|
Enzyme
Cytochrome P450
Cytochrome P450 family 21
Cytochrome P450 family 21A
Cytochrome P450 21A2
|
- | 32.4 | - | - | - | |
|
Enzyme
Cytochrome P450
Cytochrome P450 family 3
Cytochrome P450 family 3A
Cytochrome P450 3A4
|
- | 230 | - | - | 4-27 |
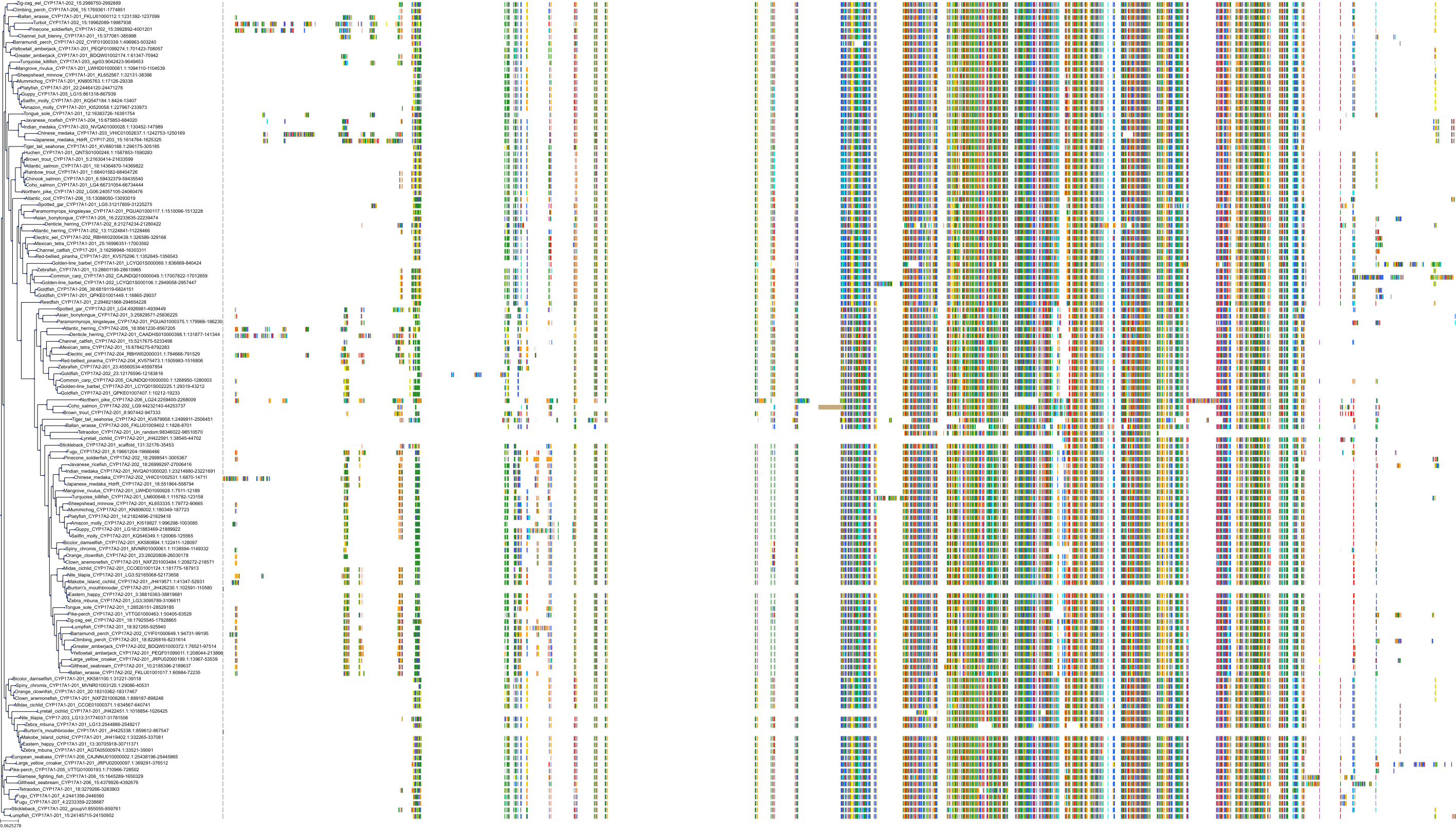
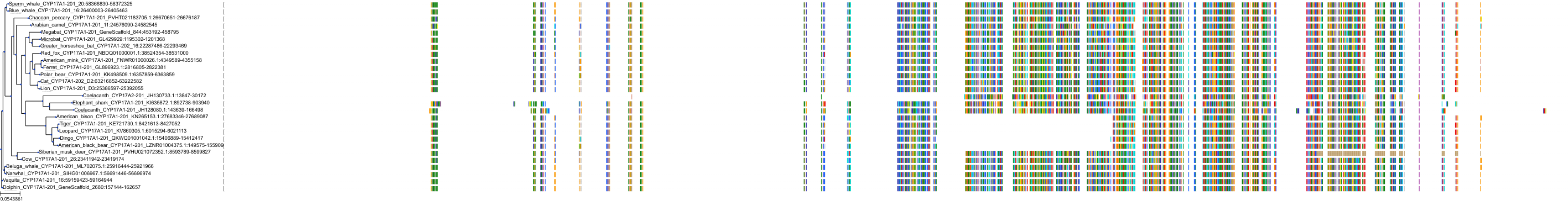
Target Conservation
|
Protein: Cytochrome P450 17A1 Description: Steroid 17-alpha-hydroxylase/17,20 lyase Organism : Homo sapiens P05093 ENSG00000148795 |
||||
|
Protein: Androgen Receptor Description: Androgen receptor Organism : Homo sapiens P10275 ENSG00000169083 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 68642 |
| ChEMBL | CHEMBL254328 |
| DrugBank | DB05812 |
| FDA SRS | G819A456D0 |
| Guide to Pharmacology | 6745 |
| PDB | AER |
| PharmGKB | PA166123407 |
| PubChem | 132971 |
| SureChEMBL | SCHEMBL61108 |
| ZINC | ZINC000003797541 |
 Homo sapiens
Homo sapiens
 Rattus norvegicus
Rattus norvegicus