Structure
| InChI Key | LIRYPHYGHXZJBZ-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C26H23FIN5O4 |
| Molecular Weight | 615.4 |
| AlogP | 3.94 |
| Hydrogen Bond Acceptor | 8.0 |
| Hydrogen Bond Donor | 2.0 |
| Number of Rotational Bond | 5.0 |
| Polar Surface Area | 107.13 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 4.0 |
| Heavy Atoms | 37.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Dual specificity mitogen-activated protein kinase kinase; MEK1/2 inhibitor | INHIBITOR | FDA Other FDA |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Enzyme
Kinase
Protein Kinase
STE protein kinase group
STE protein kinase STE7 family
|
- | 0.7-14.9 | - | - | - | |
|
Transporter
Primary active transporter
ATP-binding cassette
ABCB subfamily
|
247 | - | - | - | - |
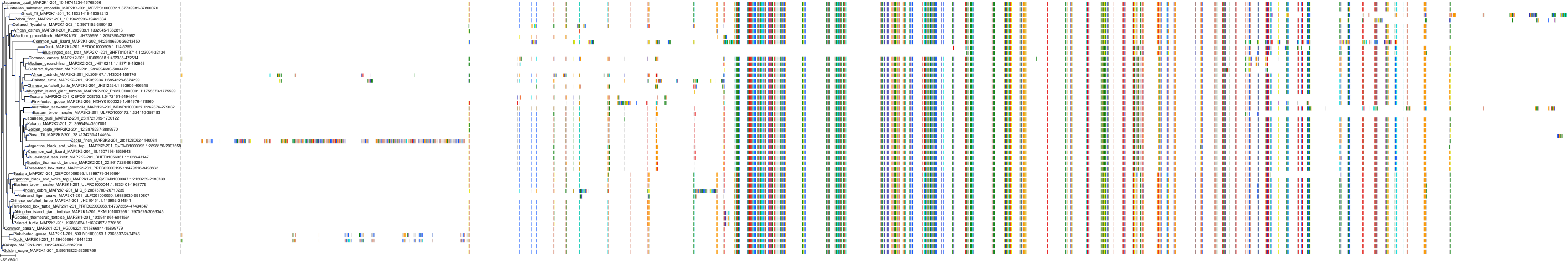
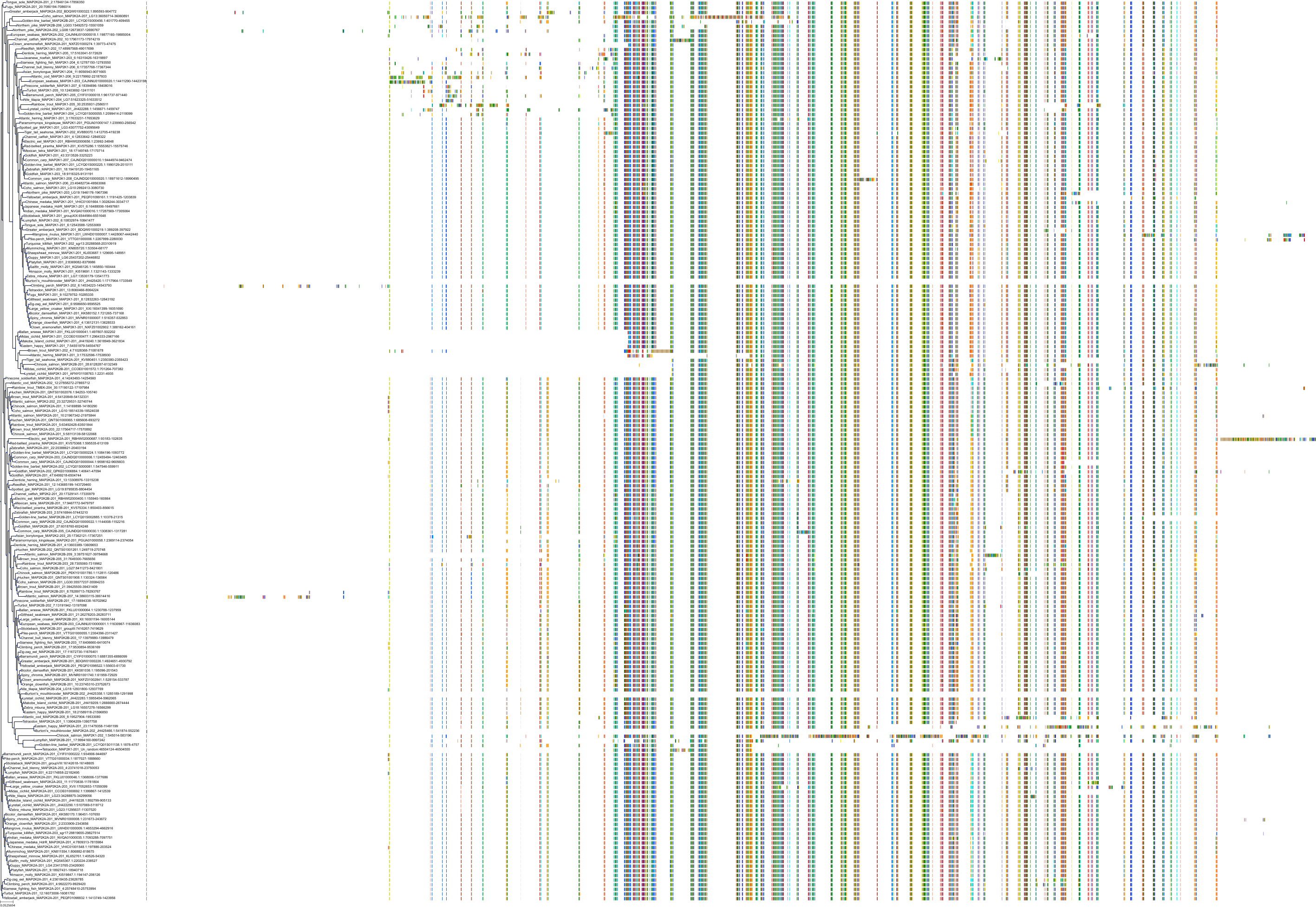
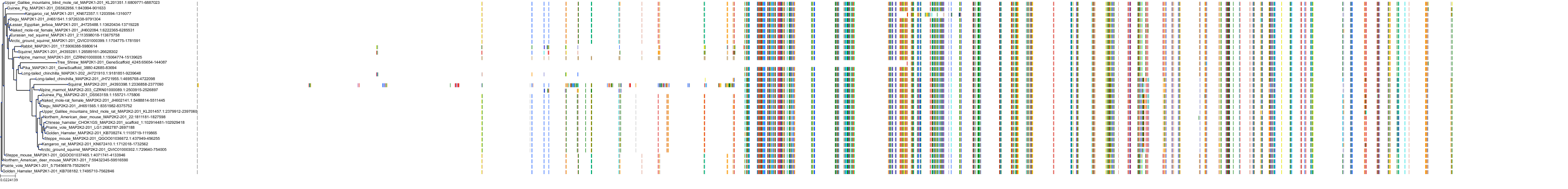
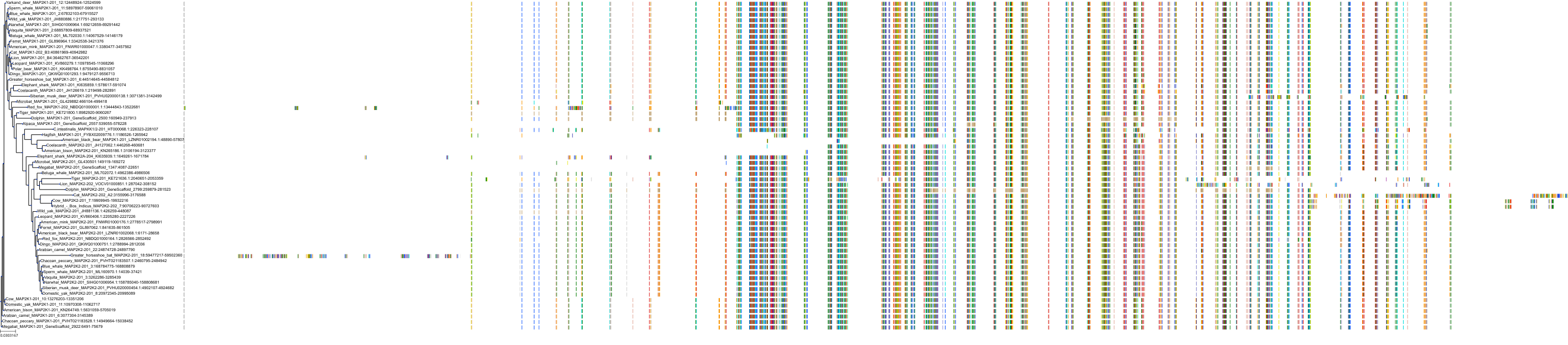
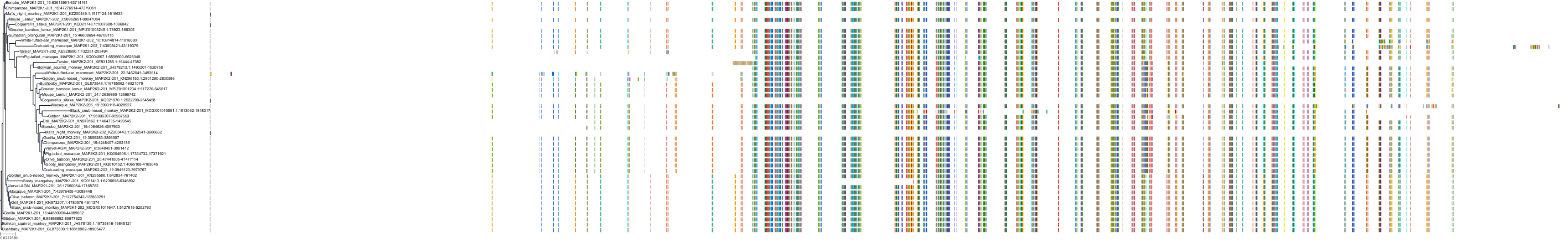
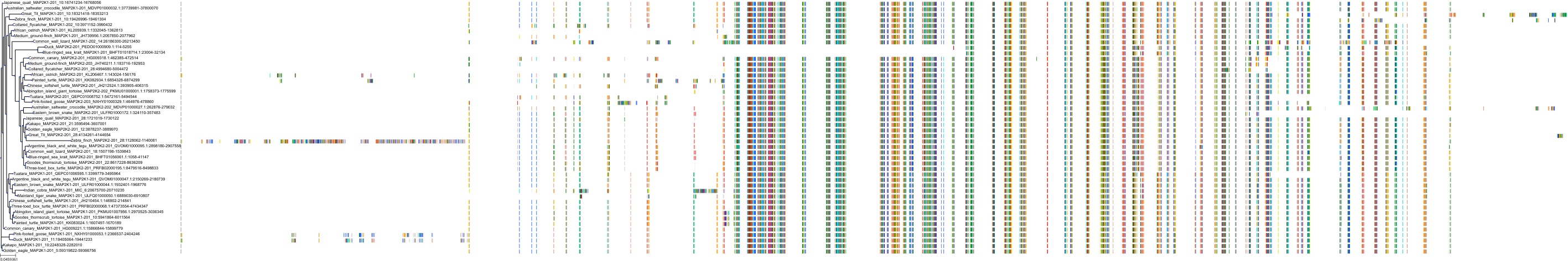
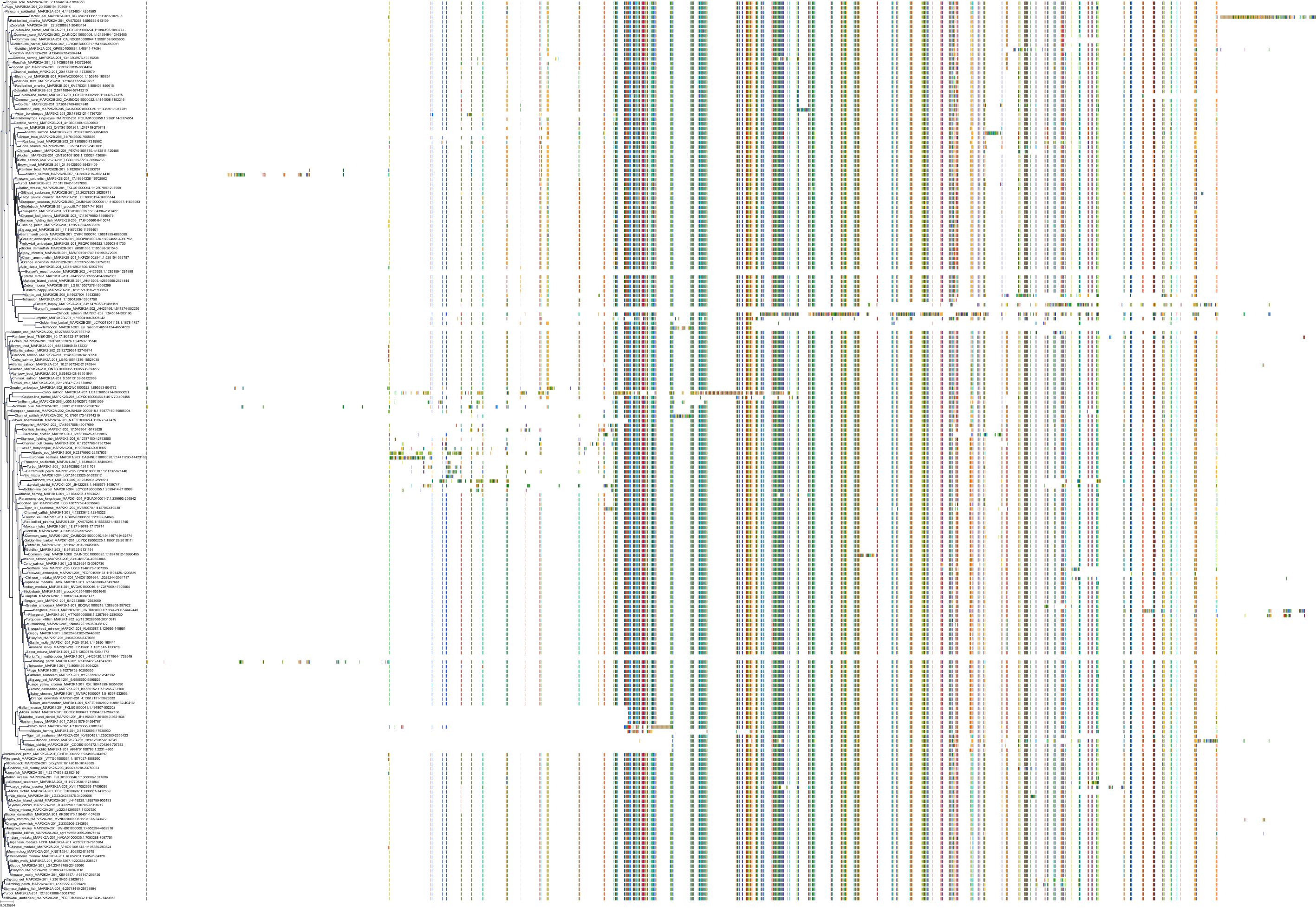
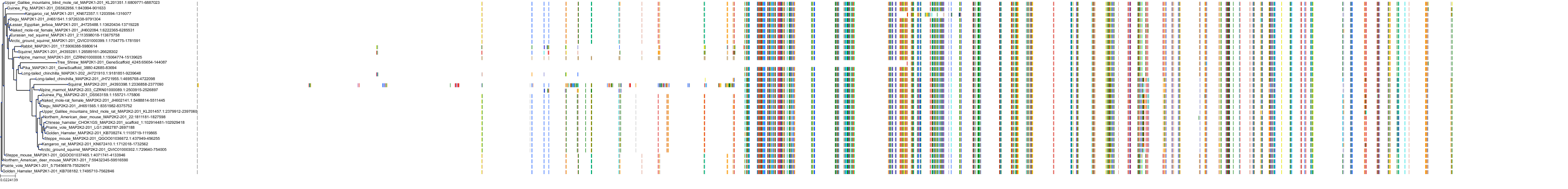
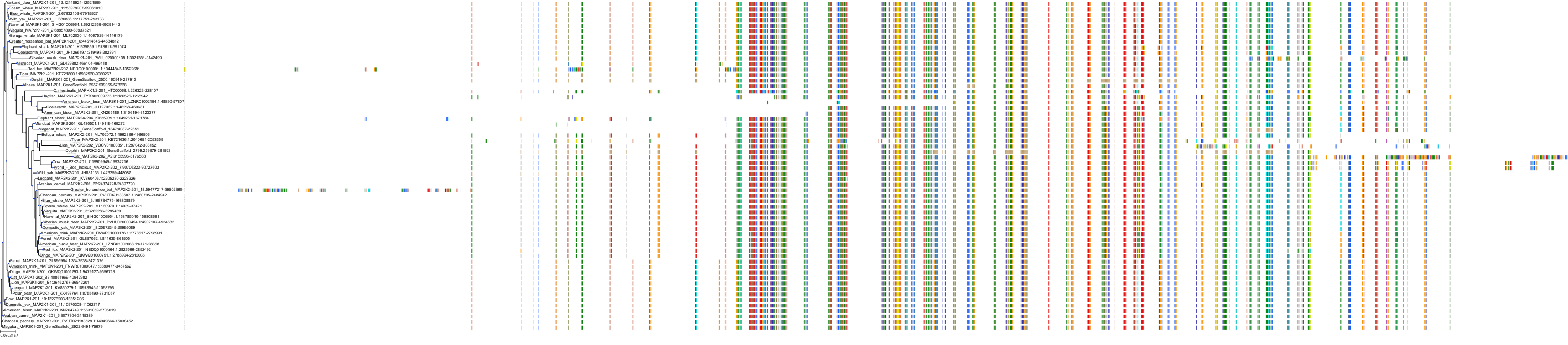
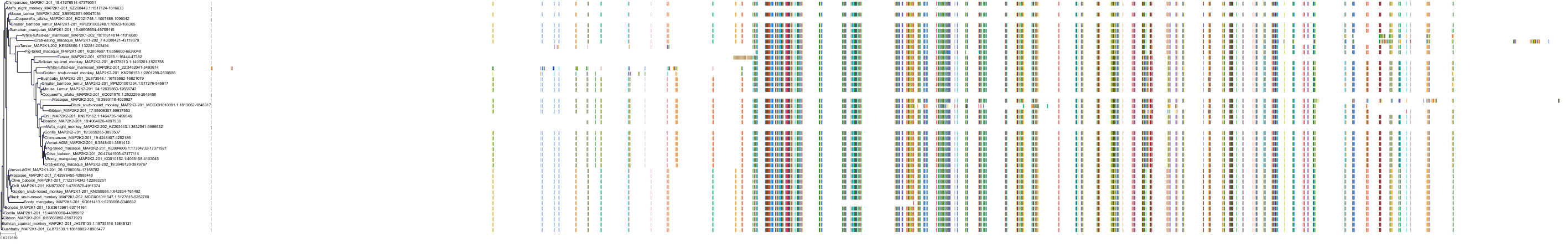
Target Conservation
|
Protein: Dual specificity mitogen-activated protein kinase kinase; MEK1/2 Description: Dual specificity mitogen-activated protein kinase kinase 2 Organism : Homo sapiens P36507 ENSG00000126934 |
||||
|
Protein: Dual specificity mitogen-activated protein kinase kinase; MEK1/2 Description: Dual specificity mitogen-activated protein kinase kinase 1 Organism : Homo sapiens Q02750 ENSG00000169032 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 75998 |
| ChEMBL | CHEMBL2103875 |
| DrugBank | DB08911 |
| DrugCentral | 4802 |
| FDA SRS | 33E86K87QN |
| Guide to Pharmacology | 6495 |
| PDB | QOM |
| PharmGKB | PA166115364 |
| PubChem | 11707110 |
| SureChEMBL | SCHEMBL170938 |
| ZINC | ZINC000043100709 |
 Homo sapiens
Homo sapiens