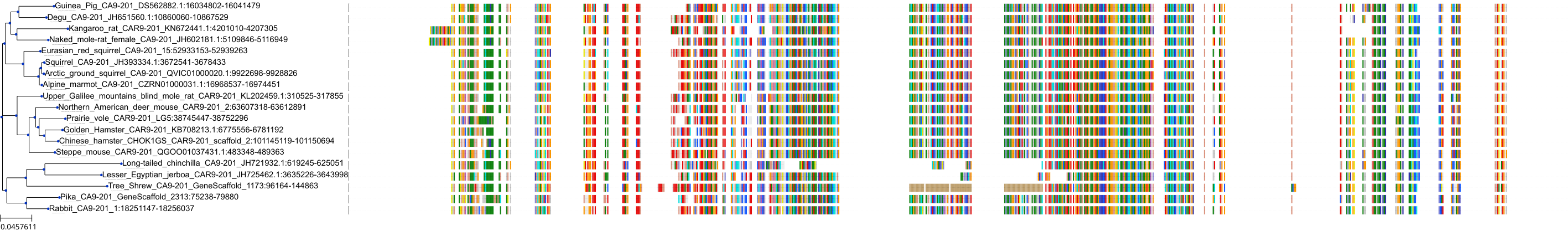
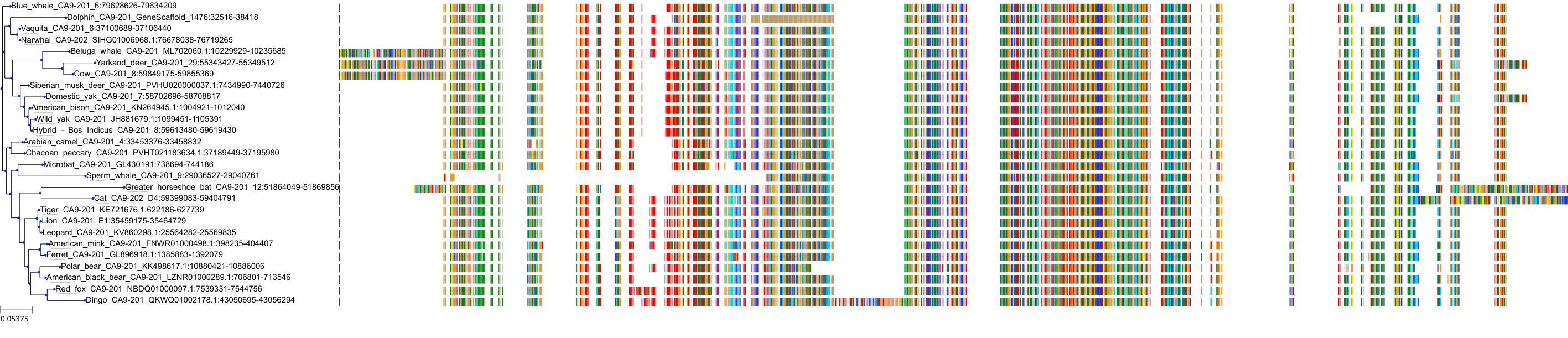
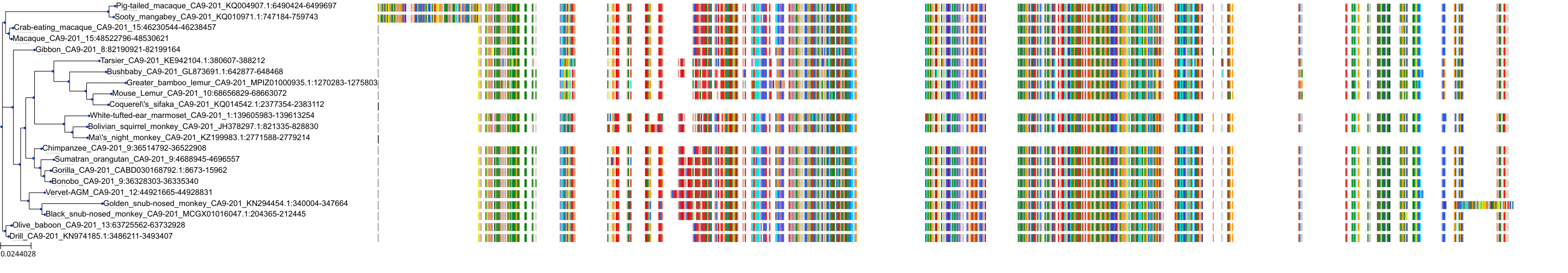
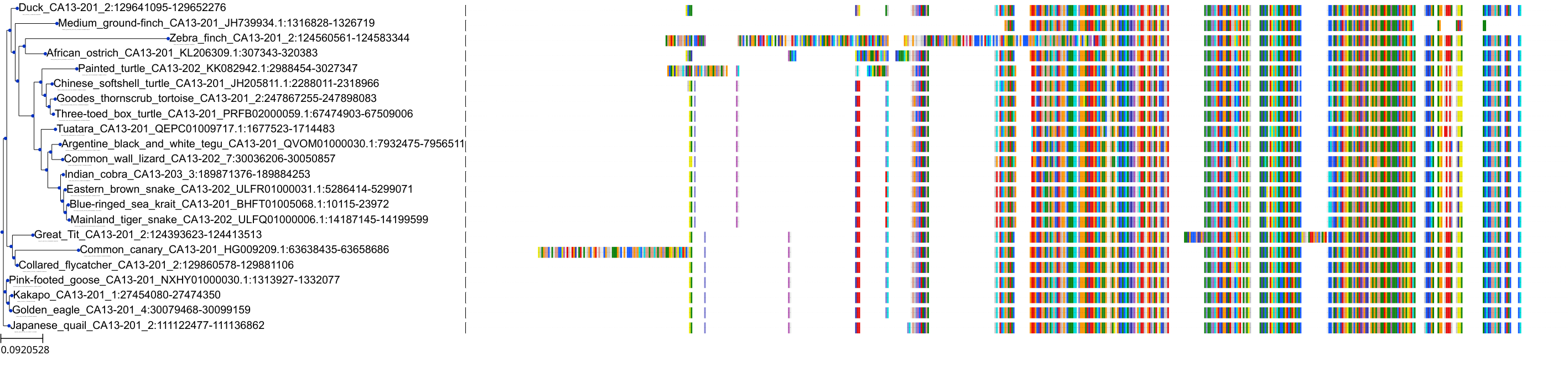
| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| UNII | I00Q766CZ2 |
| EPA CompTox | DTXSID4023626 |
Structure
| InChI Key | HMHVCUVYZFYAJI-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C10H14N2O4S2 |
| Molecular Weight | 290.37 |
| AlogP | 0.26 |
| Hydrogen Bond Acceptor | 4.0 |
| Hydrogen Bond Donor | 1.0 |
| Number of Rotational Bond | 2.0 |
| Polar Surface Area | 97.54 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 1.0 |
| Heavy Atoms | 18.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Carbonic anhydrase inhibitor | INHIBITOR | PubMed |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Enzyme
Lyase
|
- | - | - | 7-664 | - | |
|
Enzyme
|
- | - | - | 7-664 | - |
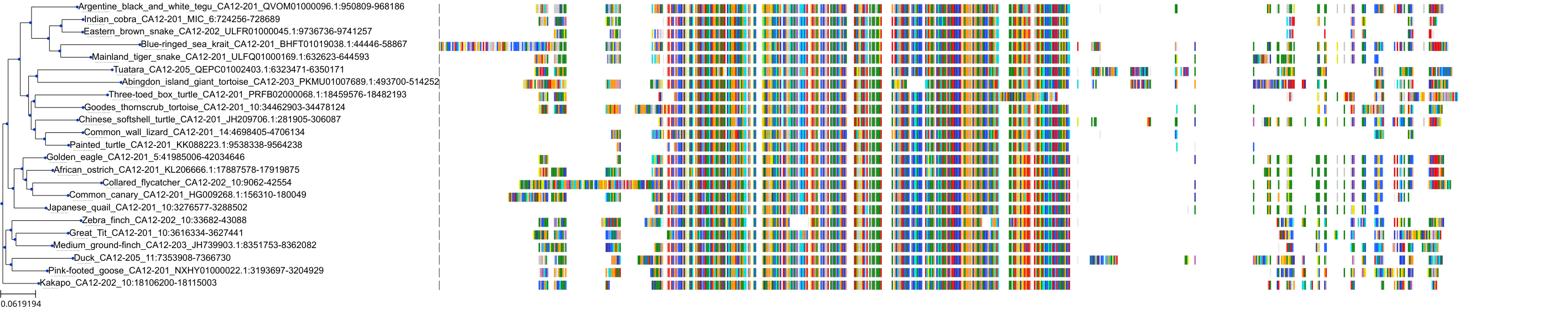
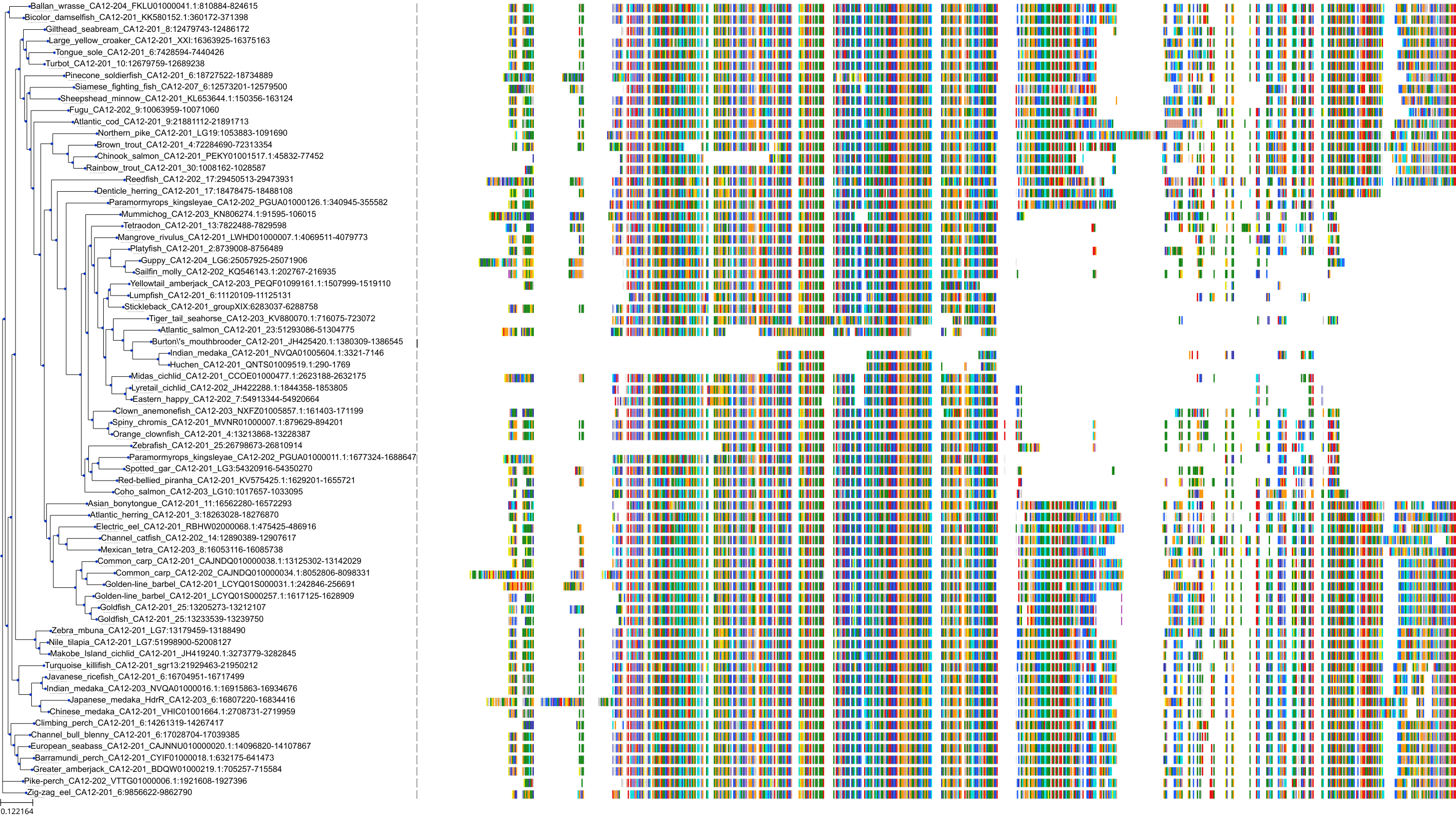
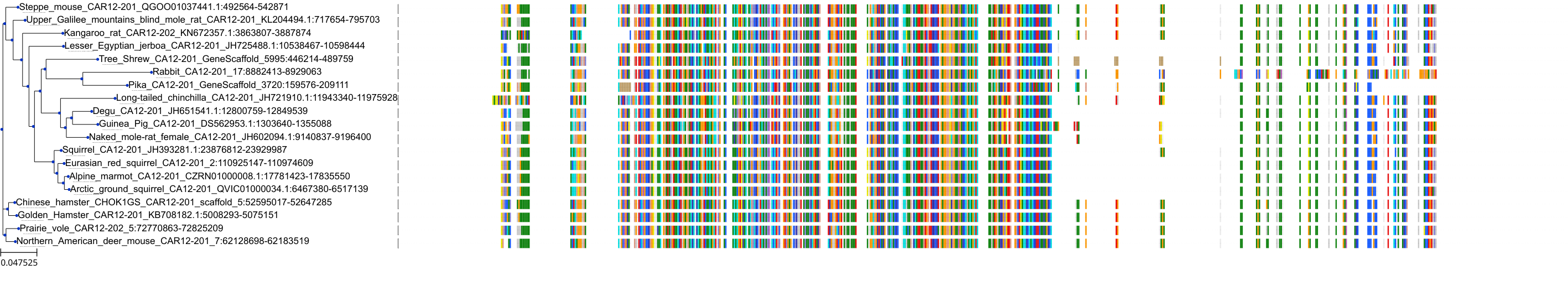
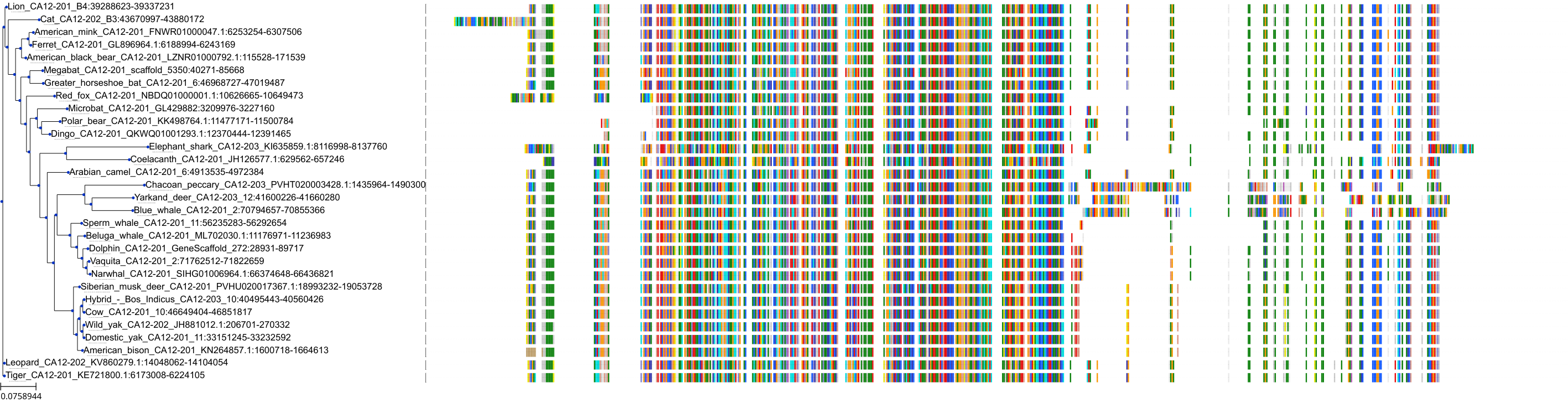
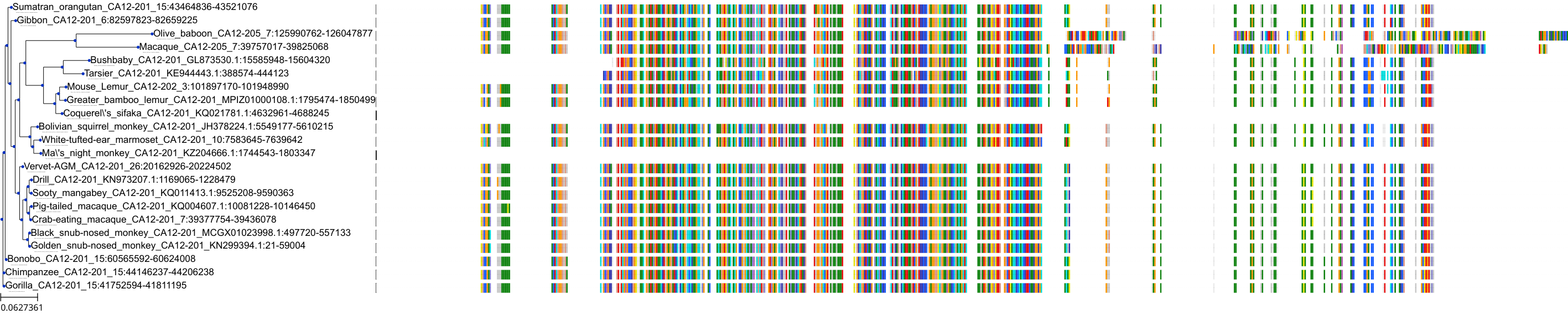
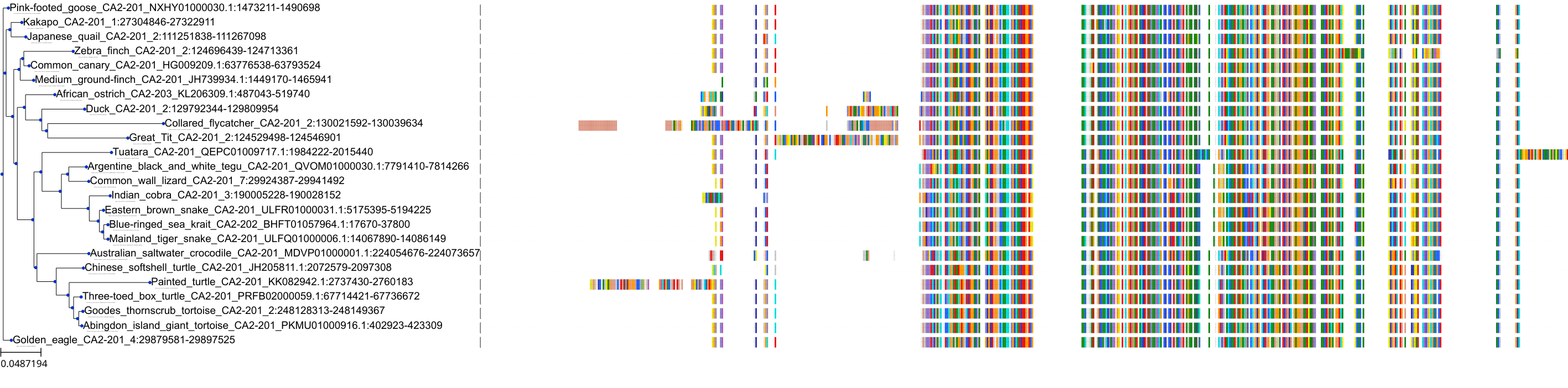
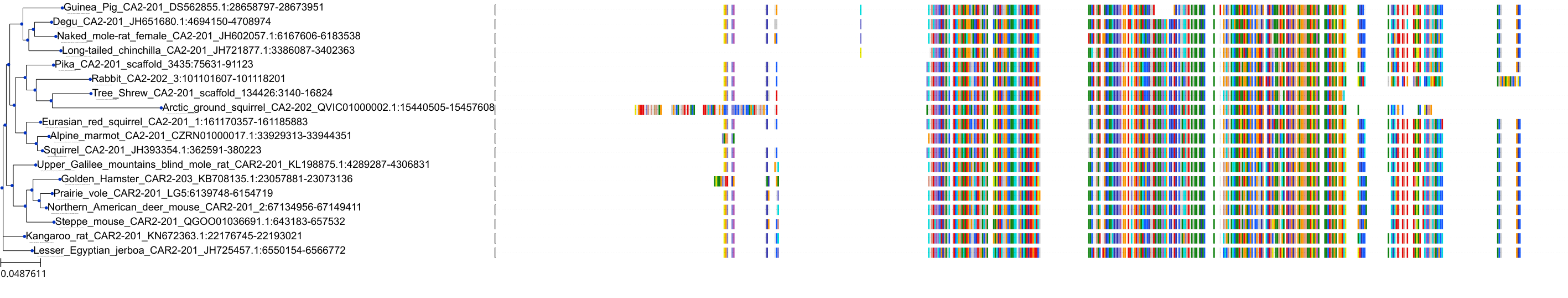
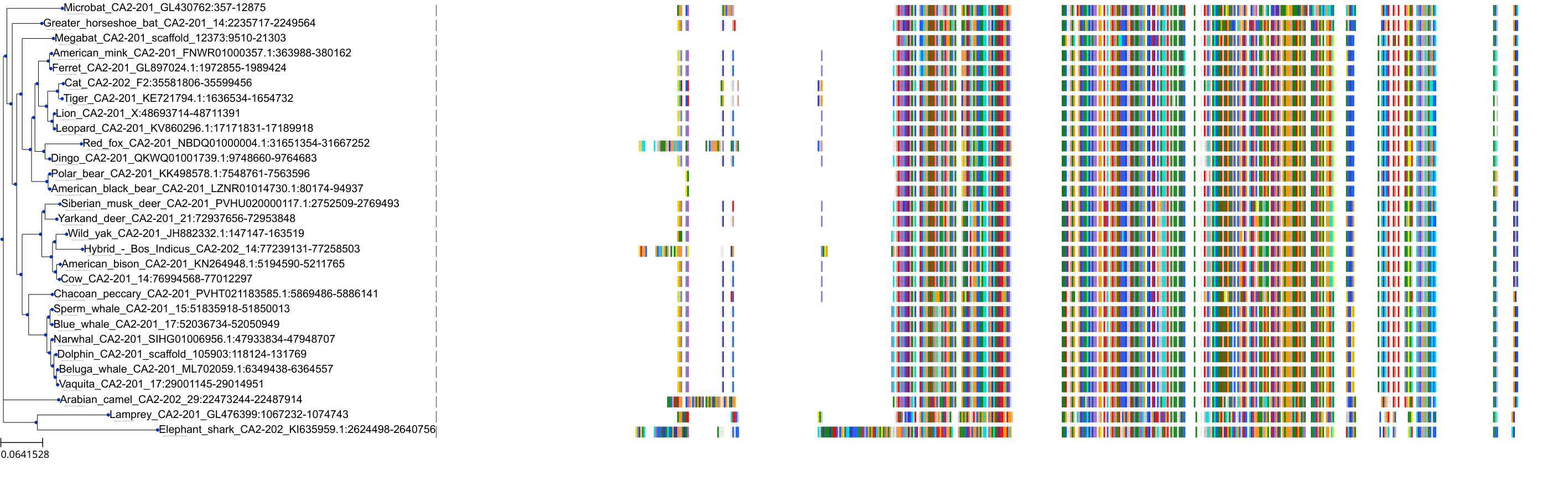
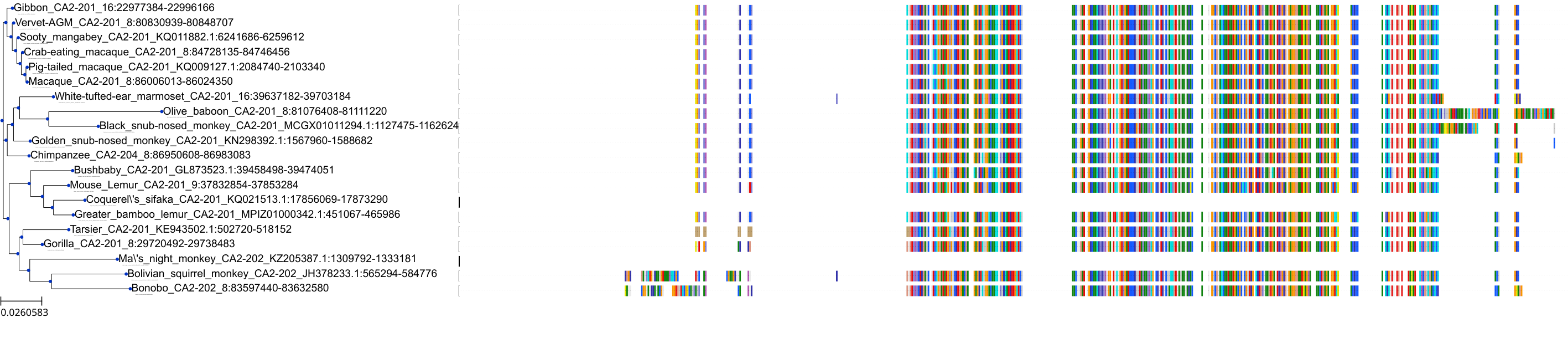
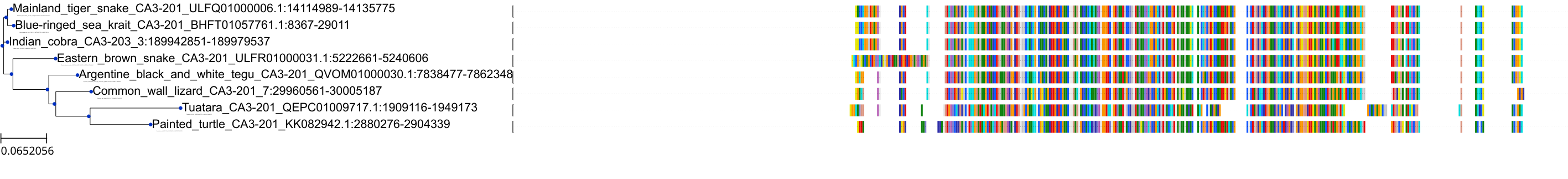
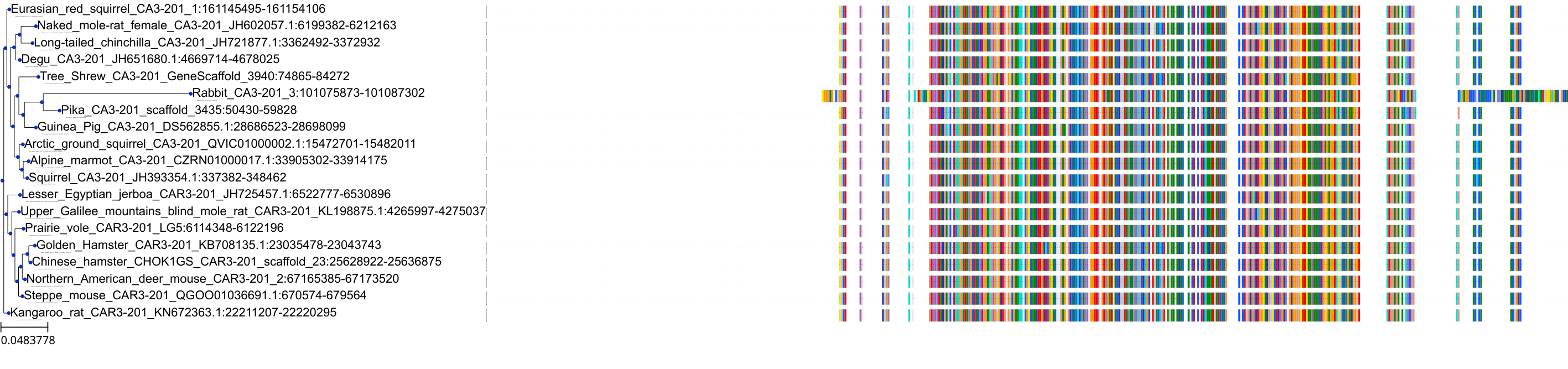
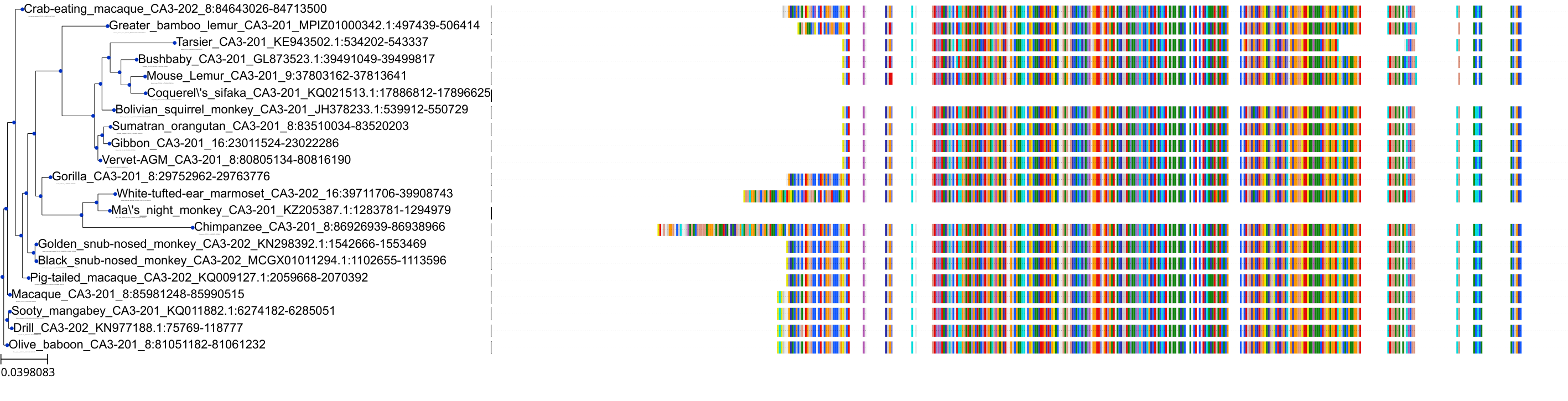
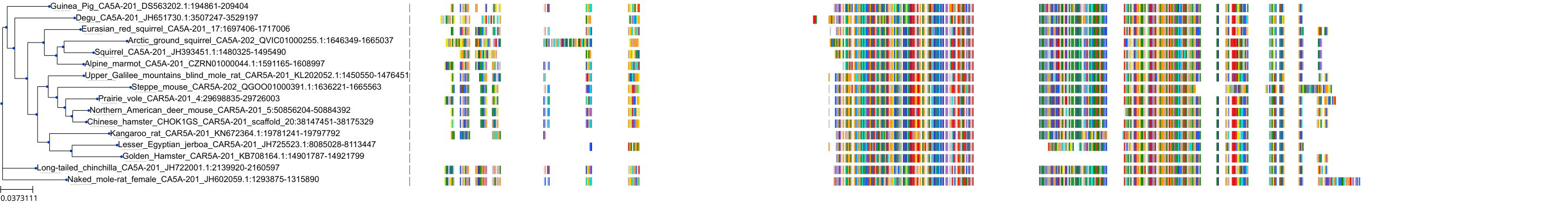
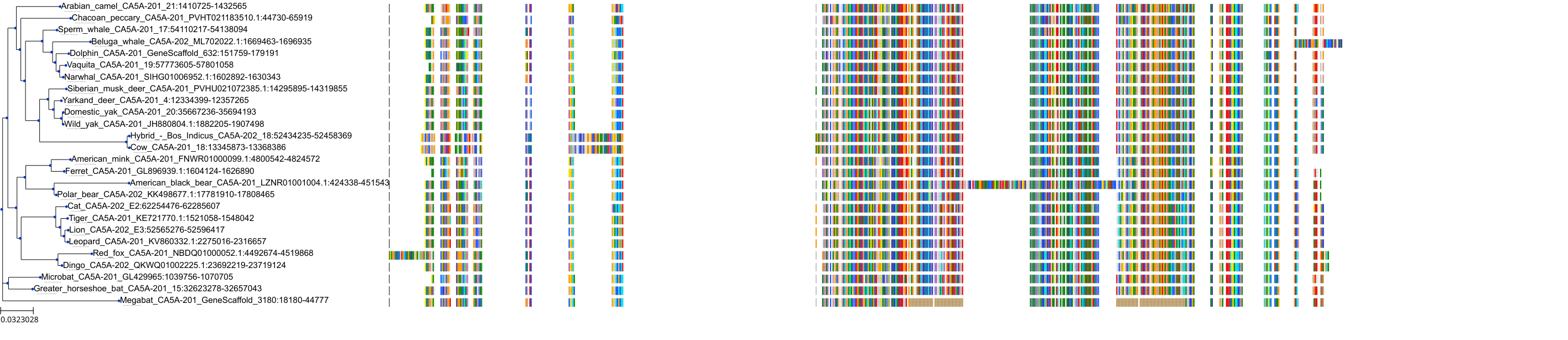
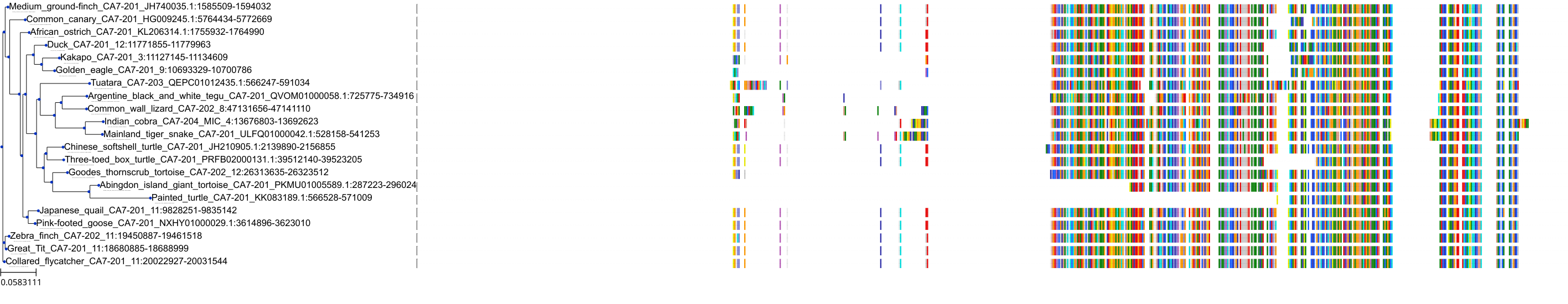
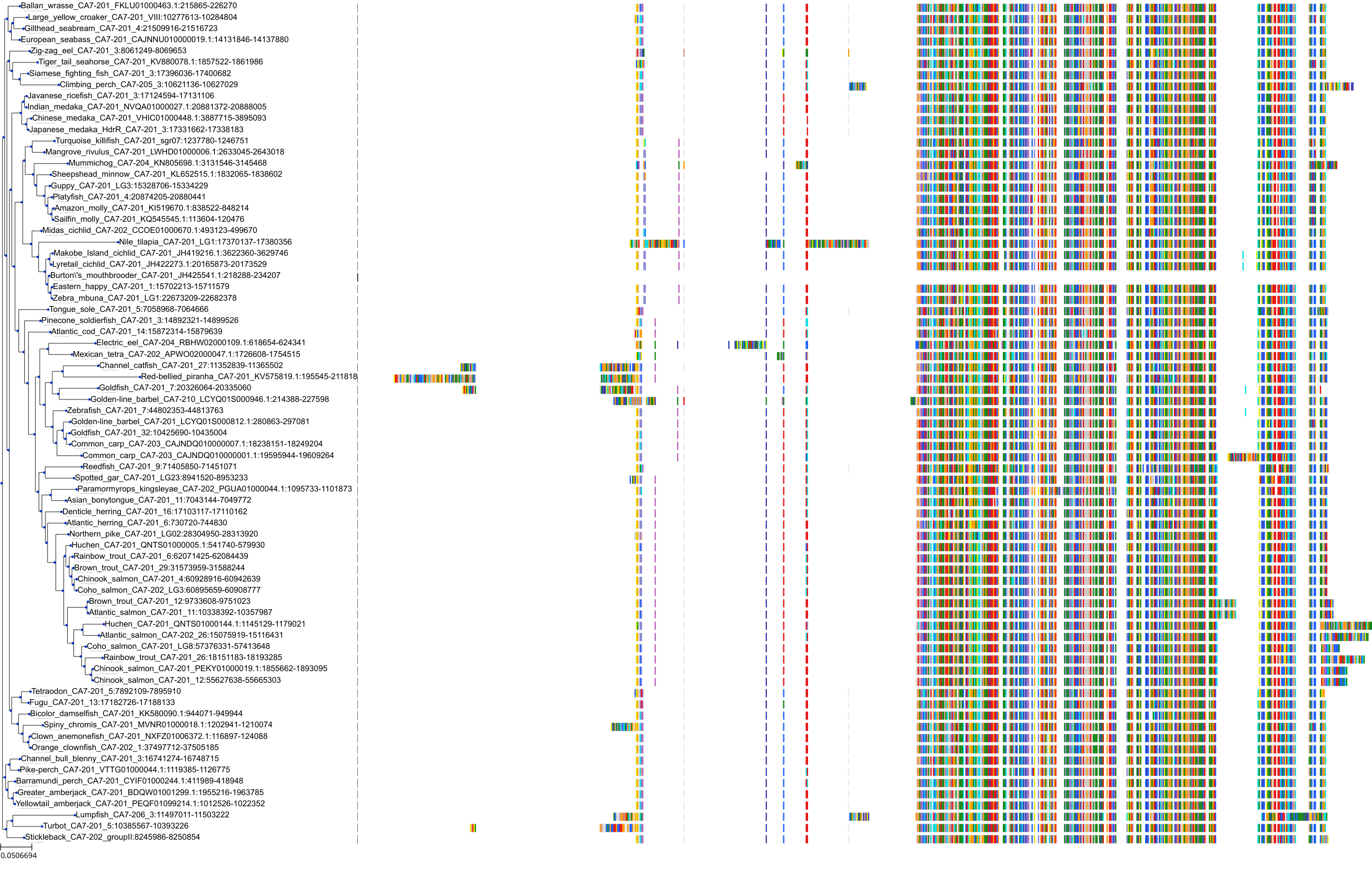
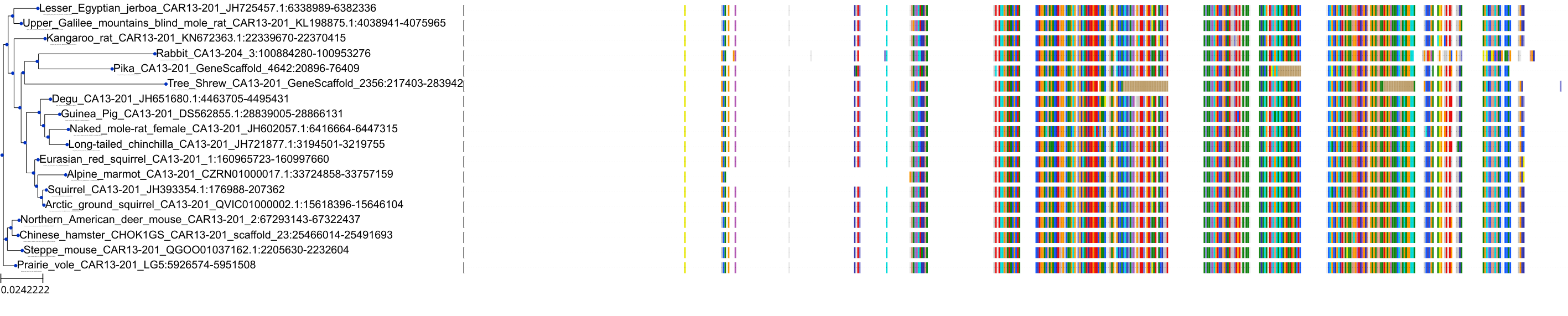
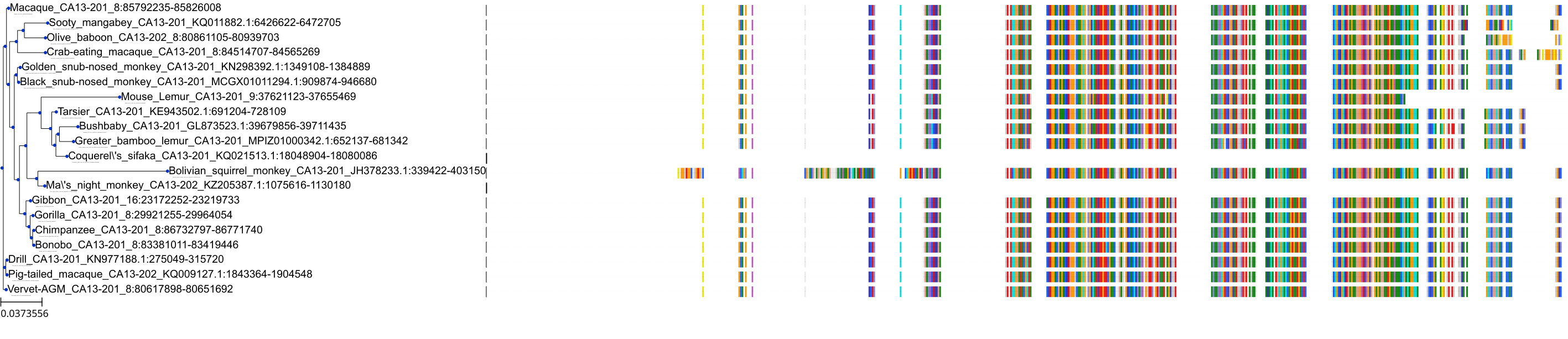
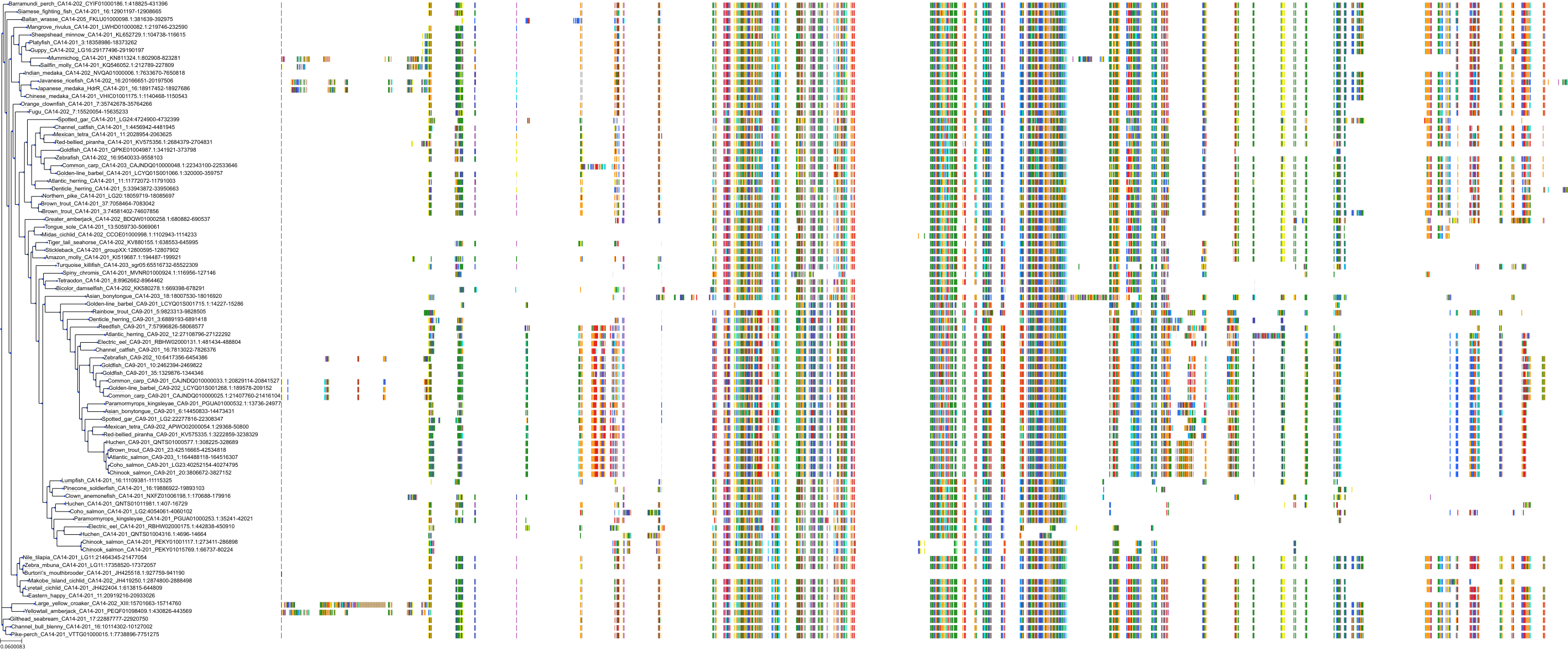
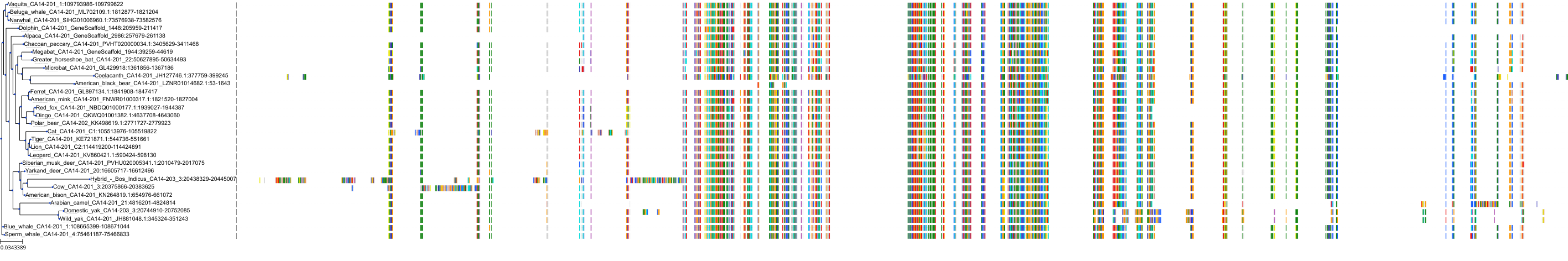
Target Conservation
|
Protein: Carbonic anhydrase Description: Carbonic anhydrase 12 Organism : Homo sapiens O43570 ENSG00000074410 |
||||
|
Protein: Carbonic anhydrase Description: Carbonic anhydrase 1 Organism : Homo sapiens P00915 ENSG00000133742 |
||||
|
Protein: Carbonic anhydrase Description: Carbonic anhydrase 2 Organism : Homo sapiens P00918 ENSG00000104267 |
||||
|
Protein: Carbonic anhydrase Description: Carbonic anhydrase 3 Organism : Homo sapiens P07451 ENSG00000164879 |
||||
|
Protein: Carbonic anhydrase Description: Carbonic anhydrase 4 Organism : Homo sapiens P22748 ENSG00000167434 |
||||
|
Protein: Carbonic anhydrase Description: Carbonic anhydrase 6 Organism : Homo sapiens P23280 ENSG00000131686 |
||||
|
Protein: Carbonic anhydrase Description: Carbonic anhydrase 5A, mitochondrial Organism : Homo sapiens P35218 ENSG00000174990 |
||||
|
Protein: Carbonic anhydrase Description: Carbonic anhydrase 7 Organism : Homo sapiens P43166 ENSG00000168748 |
||||
|
Protein: Carbonic anhydrase Description: Carbonic anhydrase 9 Organism : Homo sapiens Q16790 ENSG00000107159 |
||||
|
Protein: Carbonic anhydrase Description: Carbonic anhydrase 13 Organism : Homo sapiens Q8N1Q1 ENSG00000185015 |
||||
|
Protein: Carbonic anhydrase Description: Carbonic anhydrase 14 Organism : Homo sapiens Q9ULX7 ENSG00000118298 |
||||
|
Protein: Carbonic anhydrase Description: Carbonic anhydrase 5B, mitochondrial Organism : Homo sapiens Q9Y2D0 ENSG00000169239 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 32171 |
| ChEMBL | CHEMBL328560 |
| DrugBank | DB08329 |
| DrugCentral | 2540 |
| FDA SRS | I00Q766CZ2 |
| PDB | OSP |
| PubChem | 5356 |
| SureChEMBL | SCHEMBL38898 |
| ZINC | ZINC000000002119 |
 Anopheles gambiae
Anopheles gambiae
 Astrosclera willeyana
Astrosclera willeyana
 Chionodraco hamatus
Chionodraco hamatus
 Colwellia psychrerythraea
Colwellia psychrerythraea
 Cryptococcus neoformans
Cryptococcus neoformans
 Enterobacter sp.
Enterobacter sp.
 Helicobacter pylori
Helicobacter pylori
 Homo sapiens
Homo sapiens
 Legionella pneumophila subsp. pneumophila str. Philadelphia 1
Legionella pneumophila subsp. pneumophila str. Philadelphia 1
 Leishmania chagasi
Leishmania chagasi
 Leishmania donovani chagasi
Leishmania donovani chagasi
 Mus musculus
Mus musculus
 Mycobacterium tuberculosis
Mycobacterium tuberculosis
 Nostoc commune
Nostoc commune
 Plasmodium falciparum
Plasmodium falciparum
 Porphyromonas gingivalis
Porphyromonas gingivalis
 Pseudoalteromonas haloplanktis
Pseudoalteromonas haloplanktis
 Stylophora pistillata
Stylophora pistillata
 Sulfurihydrogenibium yellowstonense
Sulfurihydrogenibium yellowstonense
 Thalassiosira weissflogii
Thalassiosira weissflogii
 Thiomicrospira crunogena XCL-2
Thiomicrospira crunogena XCL-2
 Trypanosoma cruzi
Trypanosoma cruzi
 Vibrio cholerae
Vibrio cholerae