| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| ATC | C02AC06 |
| UNII | P67IM25ID8 |
| EPA CompTox | DTXSID3045194 |
Structure
| InChI Key | CQXADFVORZEARL-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C10H16N2O |
| Molecular Weight | 180.25 |
| AlogP | 1.15 |
| Hydrogen Bond Acceptor | 3.0 |
| Hydrogen Bond Donor | 1.0 |
| Number of Rotational Bond | 3.0 |
| Polar Surface Area | 33.62 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 0.0 |
| Heavy Atoms | 13.0 |
Pharmacology
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Membrane receptor
Family A G protein-coupled receptor
Small molecule receptor (family A GPCR)
Monoamine receptor
Adrenergic receptor
|
177.83-724.44 | - | - | 12.59-300 | - | |
|
Other cytosolic protein
|
- | - | - | 11.22-83.1 | - |
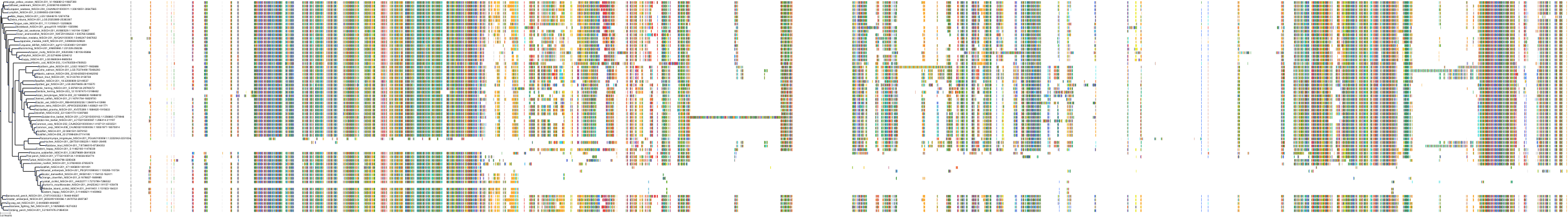
Target Conservation
|
Protein: Nischarin Description: Nischarin Organism : Homo sapiens Q9Y2I1 ENSG00000010322 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 8862 |
| ChEMBL | CHEMBL289480 |
| DrugBank | DB11738 |
| DrugCentral | 2381 |
| FDA SRS | P67IM25ID8 |
| KEGG | C11120 |
| PubChem | 68712 |
| SureChEMBL | SCHEMBL114420 |
| ZINC | ZINC000000009708 |
 Bos taurus
Bos taurus
 Homo sapiens
Homo sapiens
 Rattus norvegicus
Rattus norvegicus