| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| UNII | XKJ5VVK2WD |
| EPA CompTox | DTXSID2042640 |
Structure
| InChI Key | JLYAXFNOILIKPP-KXQOOQHDSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C47H55ClF3N5O6S3 |
| Molecular Weight | 974.63 |
| AlogP | 8.83 |
| Hydrogen Bond Acceptor | 11.0 |
| Hydrogen Bond Donor | 2.0 |
| Number of Rotational Bond | 16.0 |
| Polar Surface Area | 128.36 |
| Molecular species | ACID |
| Aromatic Rings | 4.0 |
| Heavy Atoms | 65.0 |
Pharmacology
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Enzyme
Oxidoreductase
|
- | 800 | - | - | 90.1 | |
|
Ion channel
Other ion channel
Miscellaneous ion channel
Bcl-2 family
|
- | 2-60 | 0.65-20.6 | 0.044-48 | - | |
|
Other cytosolic protein
|
- | 70-500 | - | 245-550 | - | |
|
Unclassified protein
|
- | 4.3 | - | - | - |
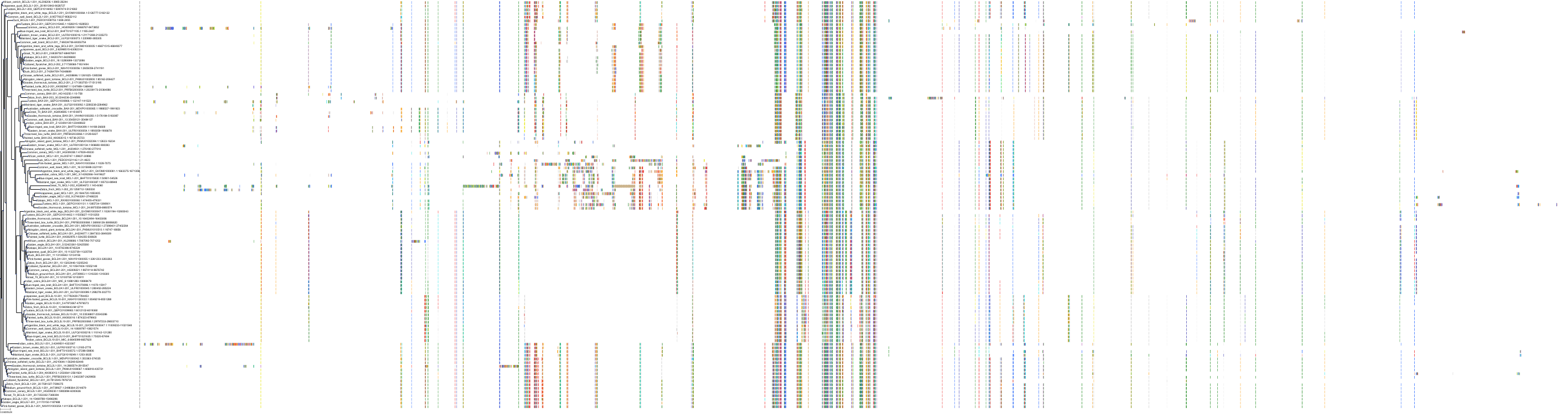
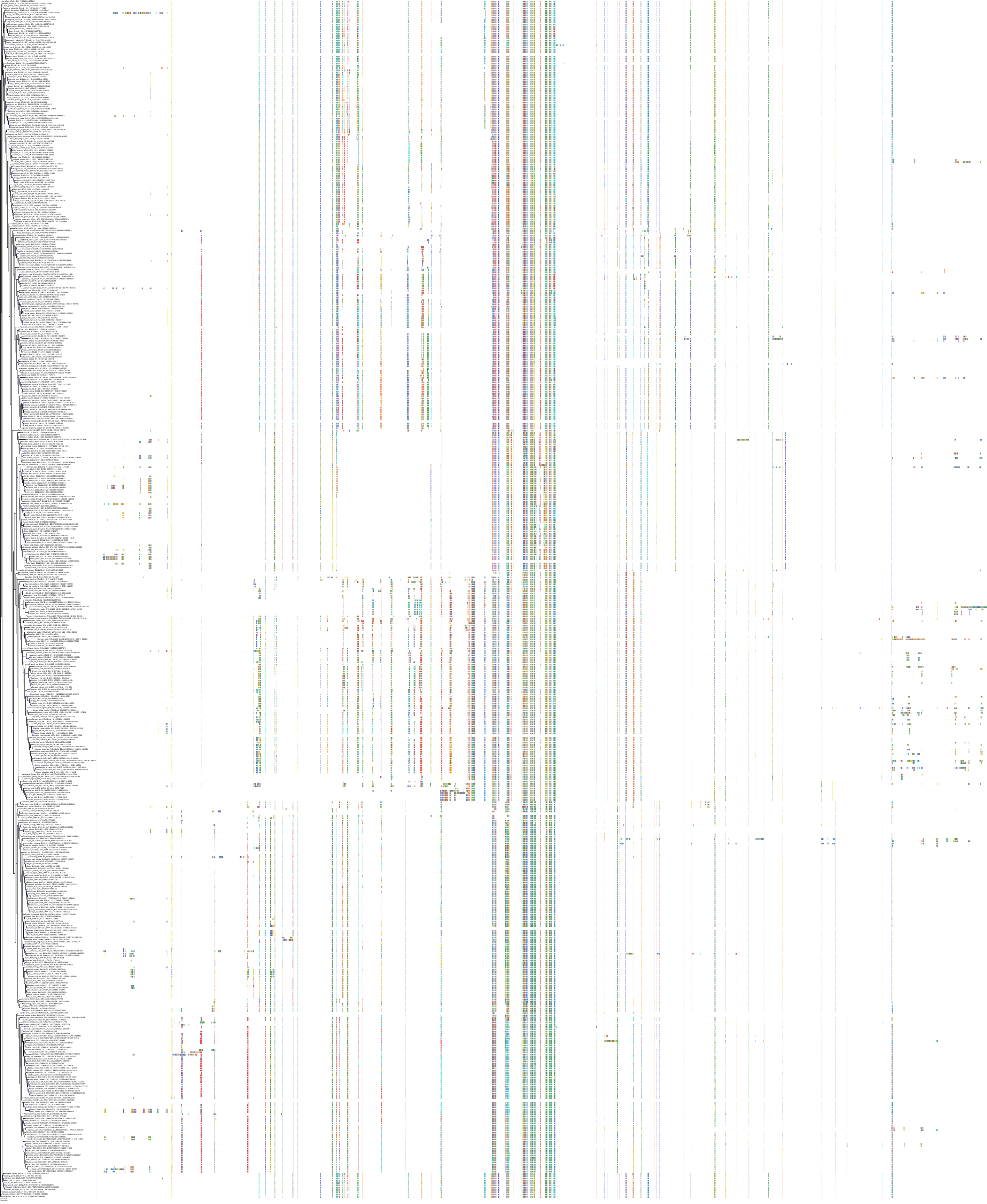
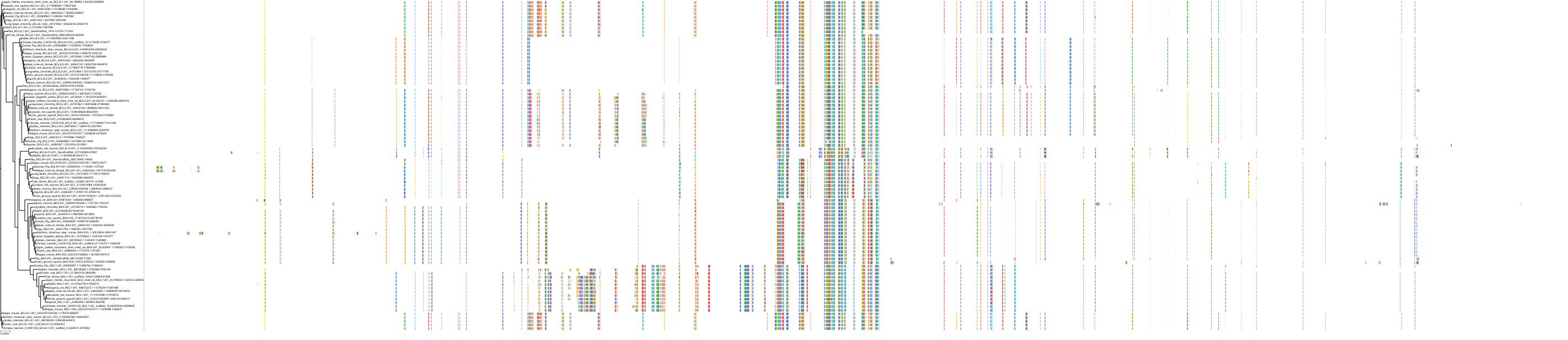
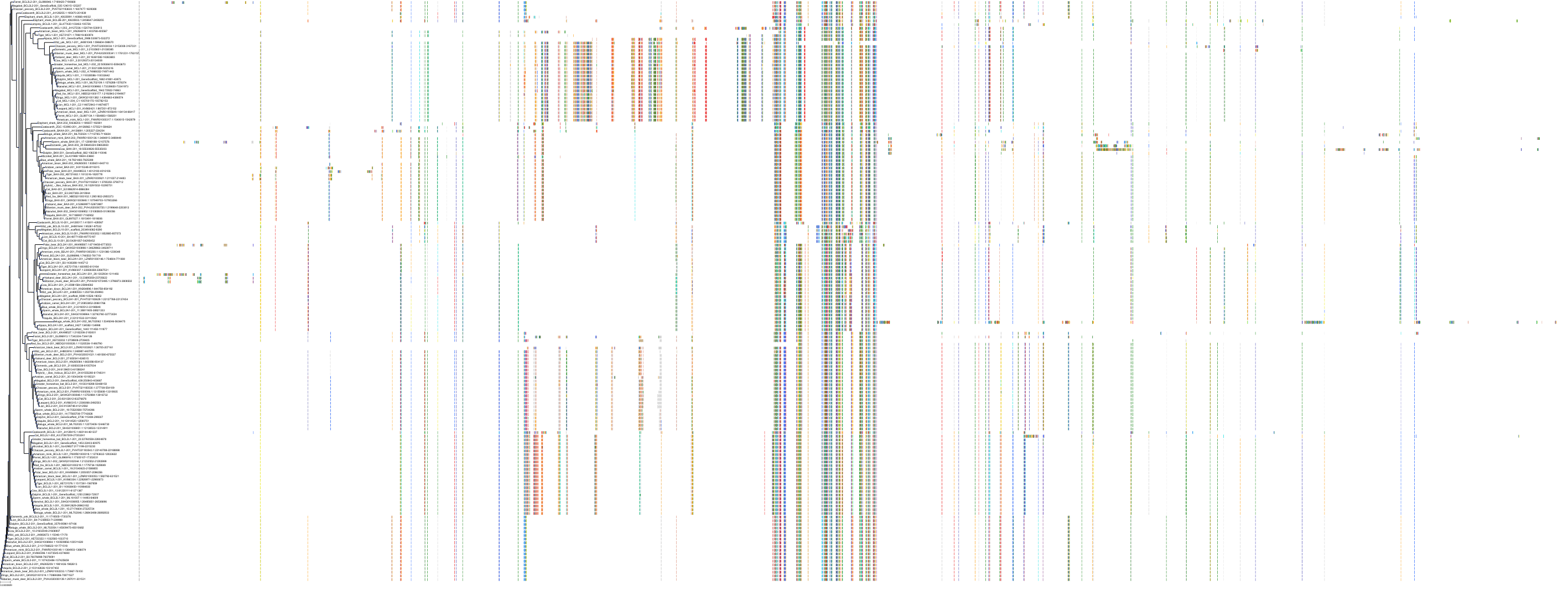
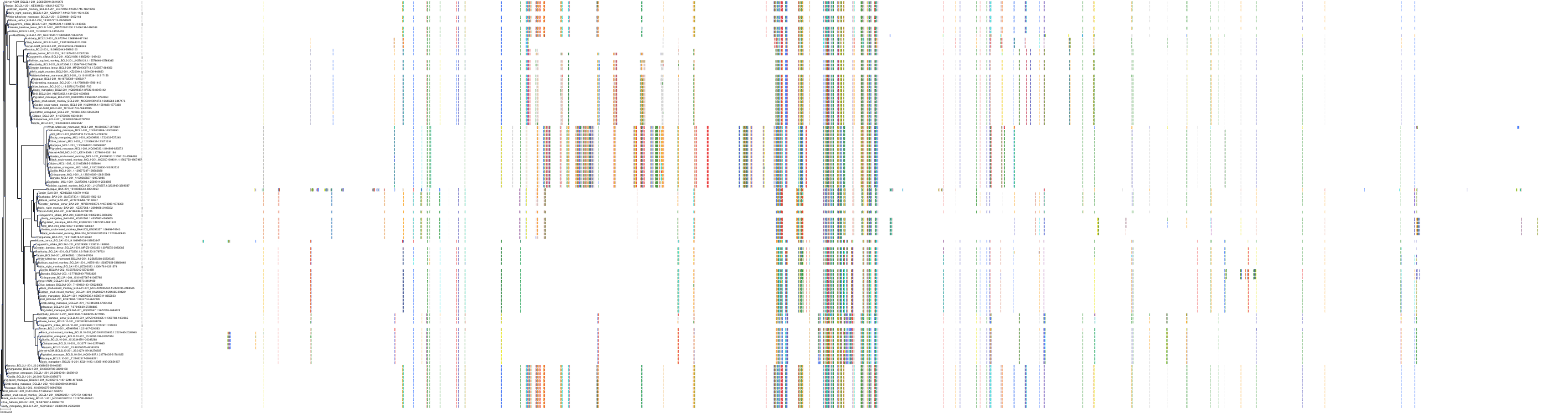
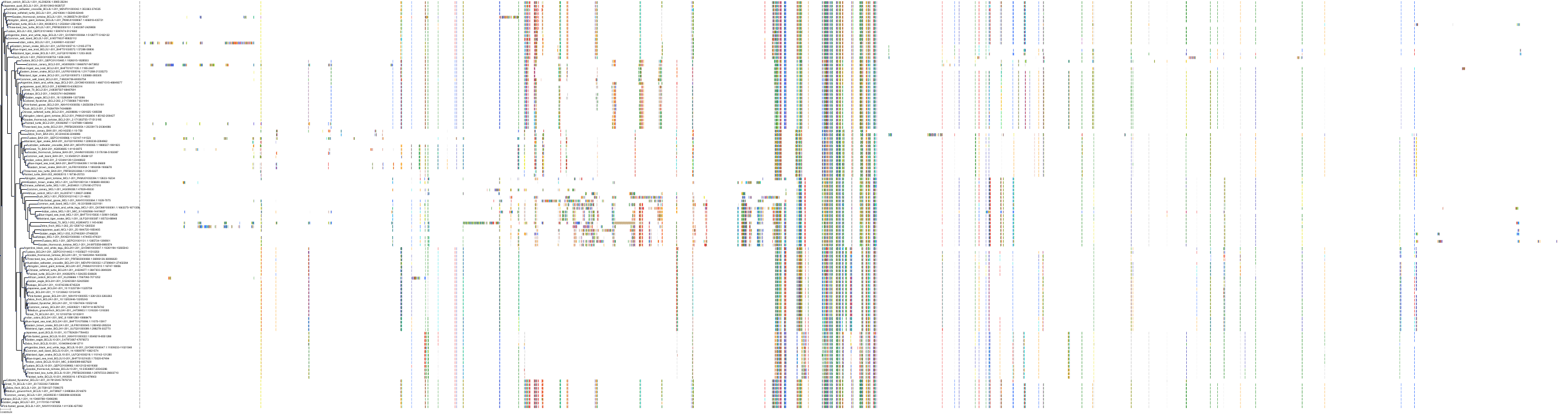
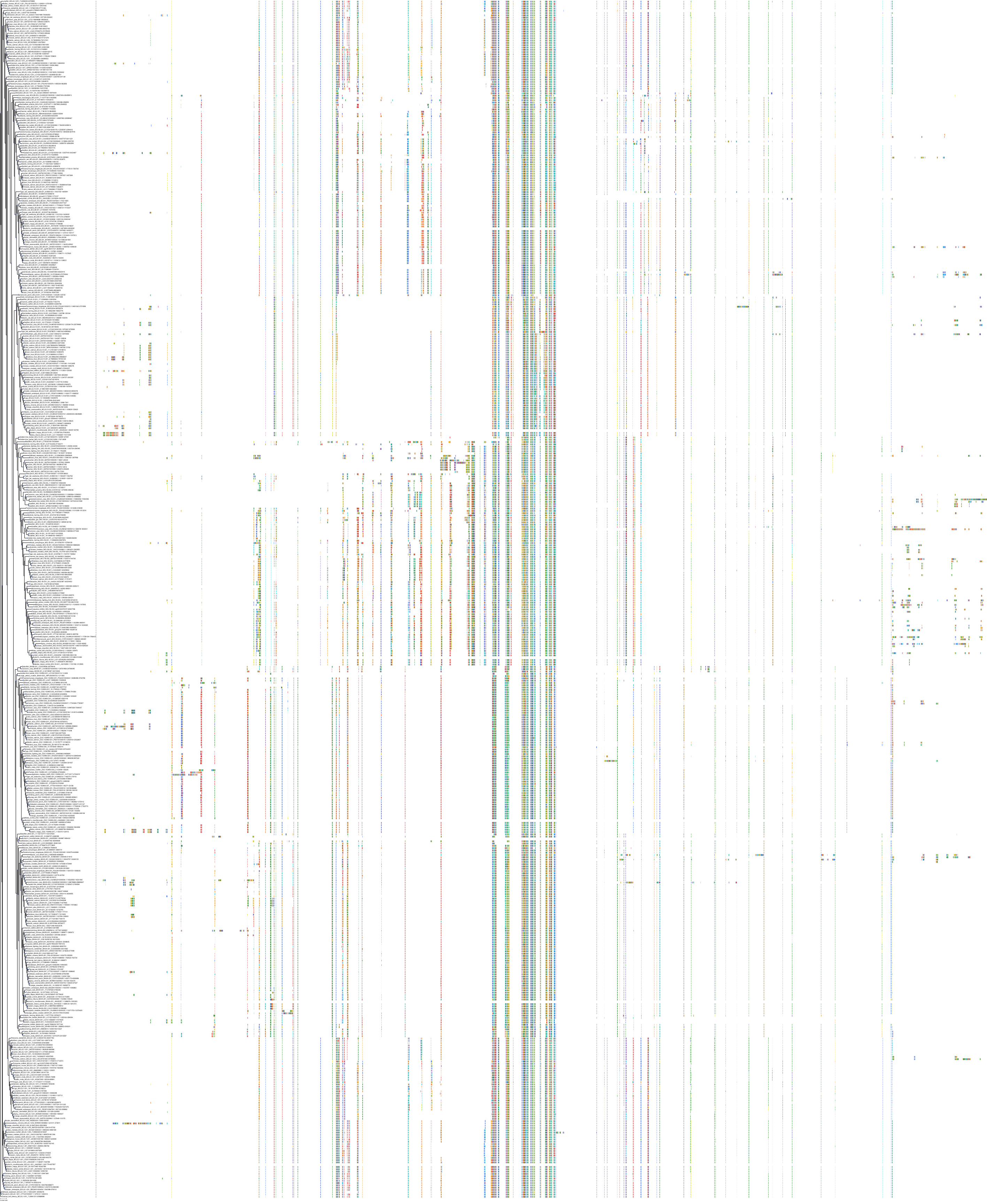
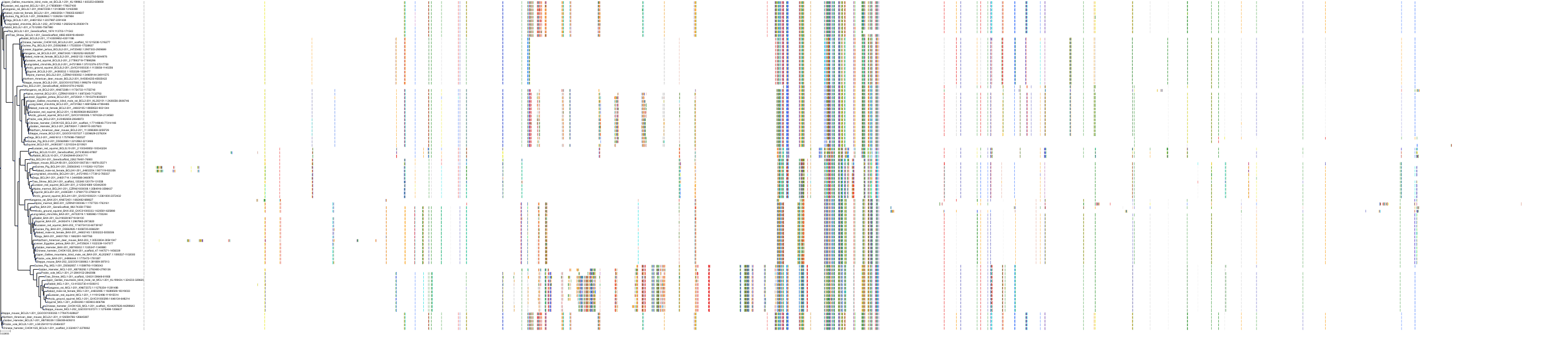
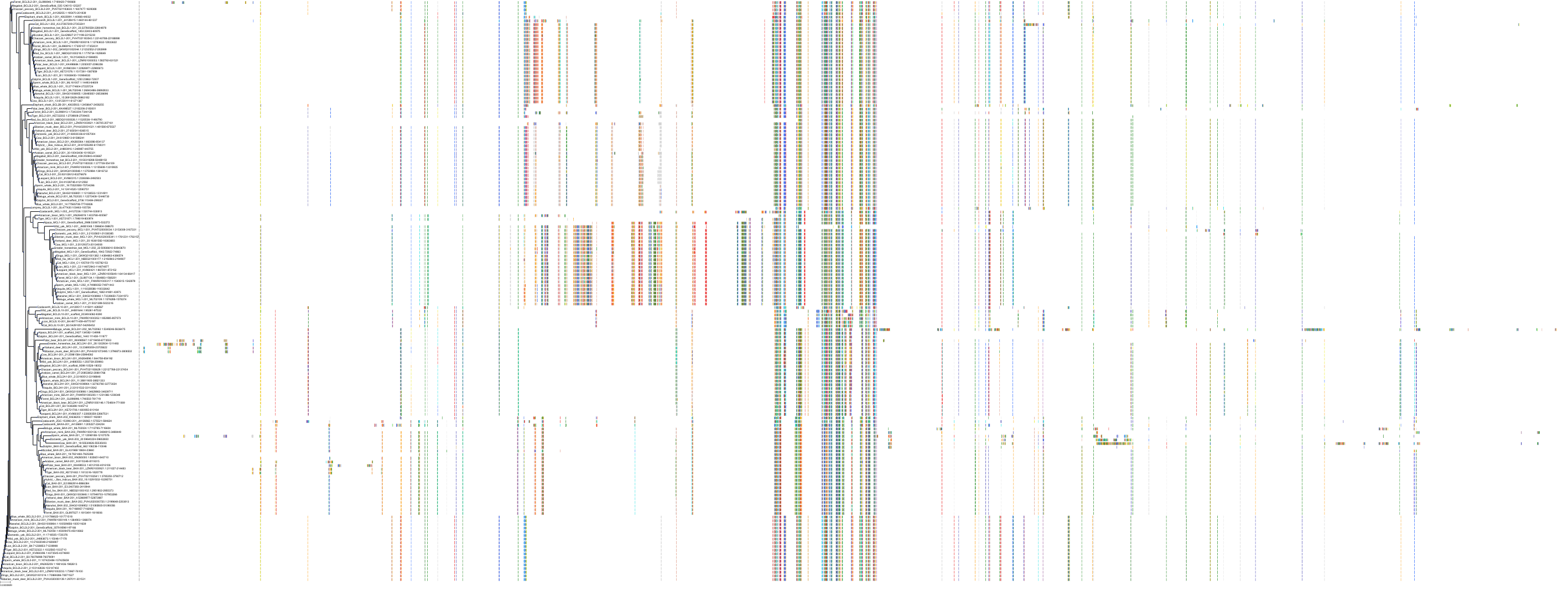
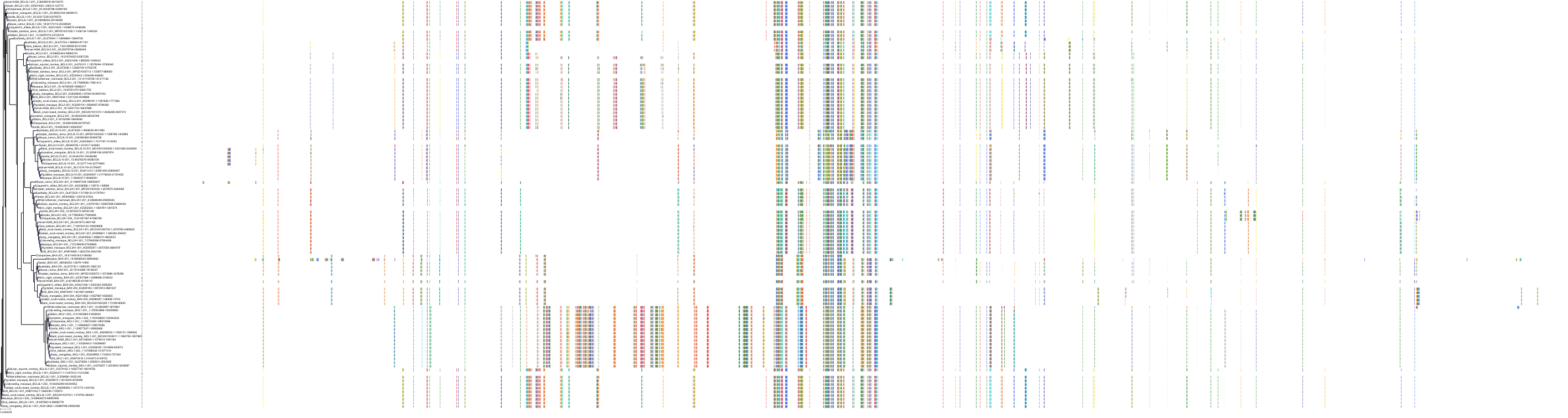
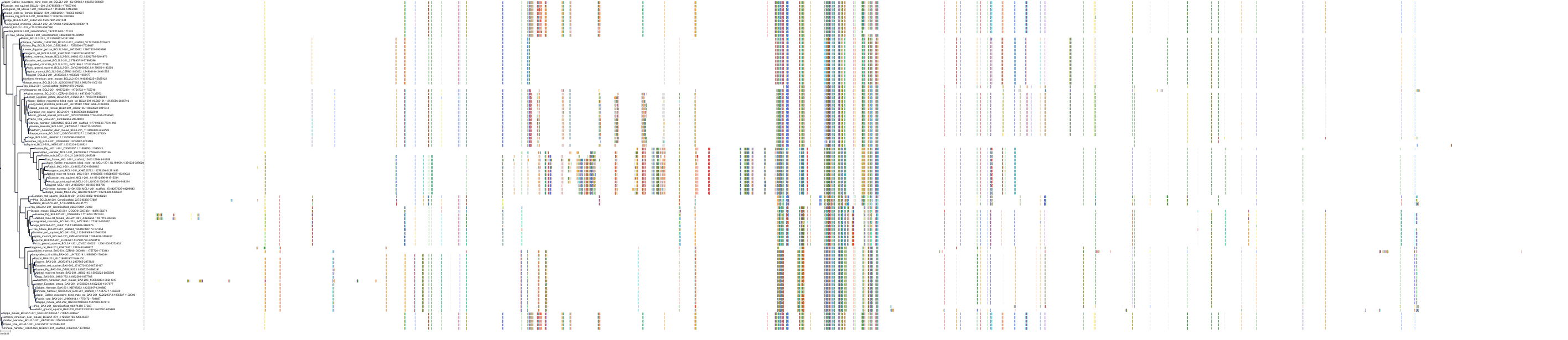
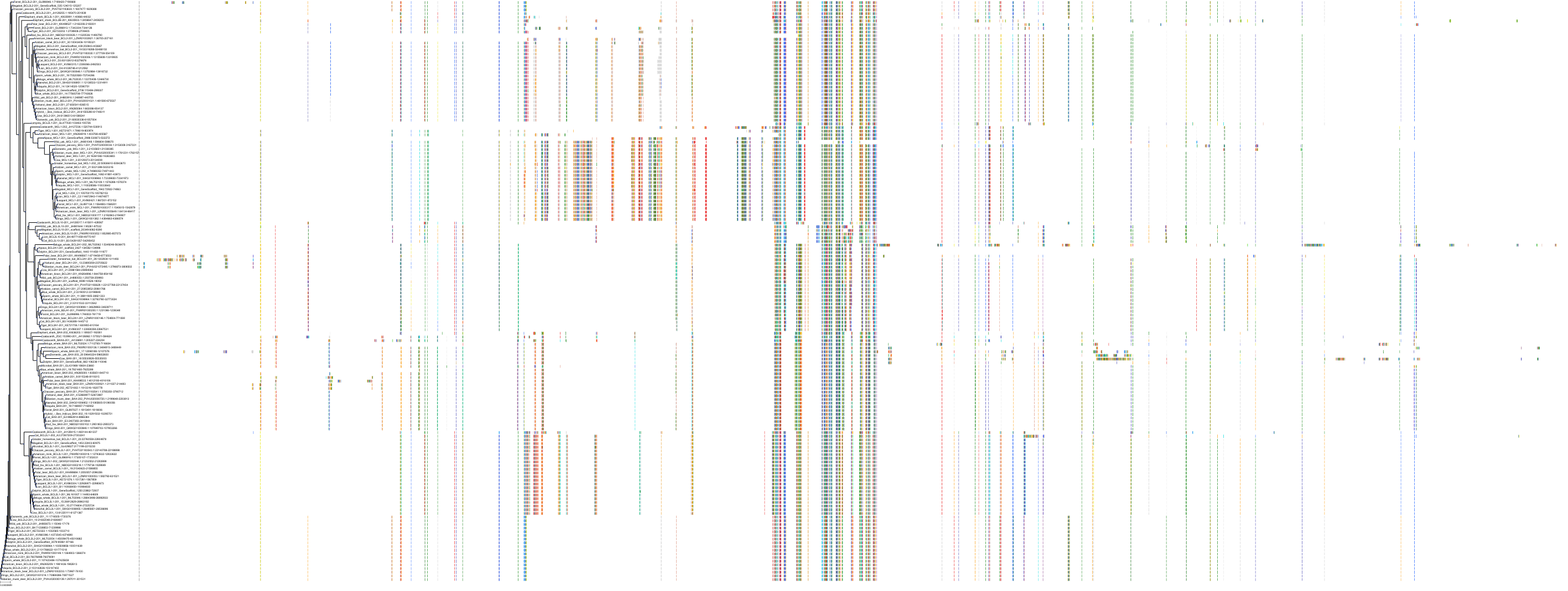
Target Conservation
|
Protein: Apoptosis regulator Bcl-2 Description: Apoptosis regulator Bcl-2 Organism : Homo sapiens P10415 ENSG00000171791 |
||||
|
Protein: Apoptosis regulator Bcl-X Description: Bcl-2-like protein 1 Organism : Homo sapiens Q07817 ENSG00000171552 |
||||
|
Protein: Apoptosis regulator Bcl-W Description: Bcl-2-like protein 2 Organism : Homo sapiens Q92843 ENSG00000129473 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 131174 |
| ChEMBL | CHEMBL443684 |
| DrugBank | DB12340 |
| FDA SRS | XKJ5VVK2WD |
| Guide to Pharmacology | 8319 |
| PDB | 1XJ |
| PubChem | 24978538 |
| SureChEMBL | SCHEMBL522847 |
| ZINC | ZINC000150338726 |
 Homo sapiens
Homo sapiens
 Mus musculus
Mus musculus