| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| UNII | A6GWB8T96J |
| EPA CompTox | DTXSID80222945 |
Structure
| InChI Key | HRNLUBSXIHFDHP-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C23H20N6O |
| Molecular Weight | 396.45 |
| AlogP | 3.99 |
| Hydrogen Bond Acceptor | 6.0 |
| Hydrogen Bond Donor | 3.0 |
| Number of Rotational Bond | 6.0 |
| Polar Surface Area | 105.82 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 4.0 |
| Heavy Atoms | 30.0 |
Pharmacology
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Enzyme
Cytochrome P450
Cytochrome P450 family 3
Cytochrome P450 family 3A
Cytochrome P450 3A4
|
- | 570 | - | - | - | |
|
Epigenetic regulator
Eraser
Histone deacetylase
HDAC class I
|
- | 22-950 | - | 9-265 | 80 | |
|
Epigenetic regulator
Eraser
Histone deacetylase
HDAC class IIb
|
- | - | - | - | 32-68 | |
|
Epigenetic regulator
Eraser
Histone deacetylase
HDAC class IV
|
- | 590-600 | - | - | - | |
|
Epigenetic regulator
Writer
Histone acetyltransferase
SRC family
|
- | 22 | - | - | - | |
|
Ion channel
Voltage-gated ion channel
Potassium channels
Voltage-gated potassium channel
|
- | - | - | - | 30 | |
|
Unclassified protein
|
- | 45 | - | - | - |
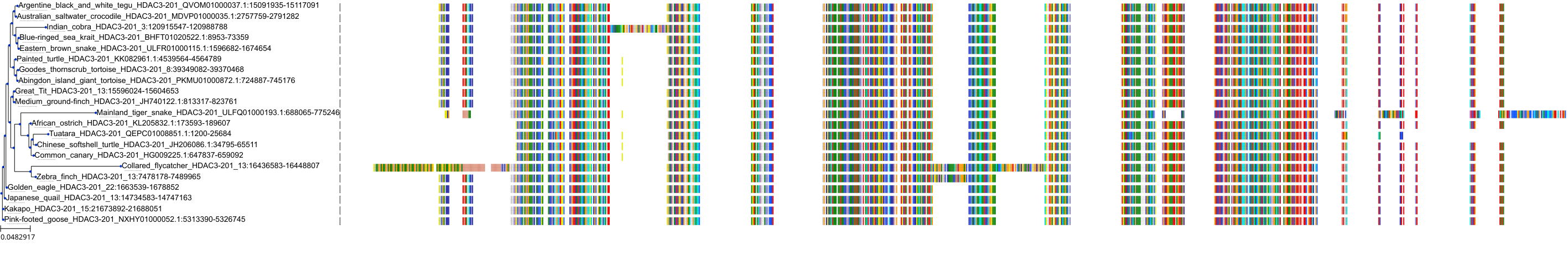
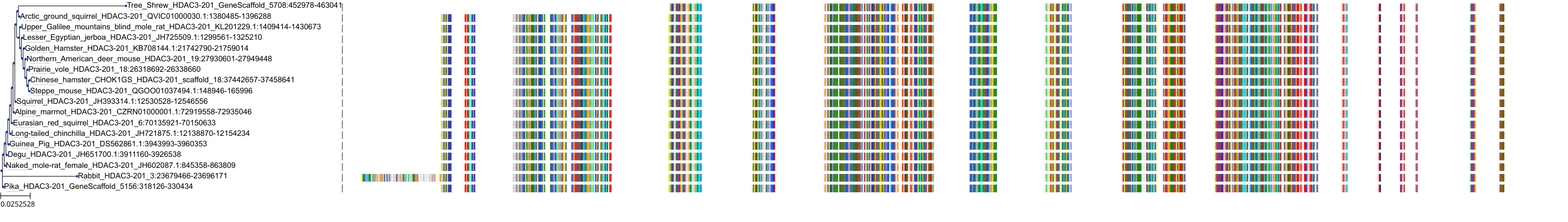
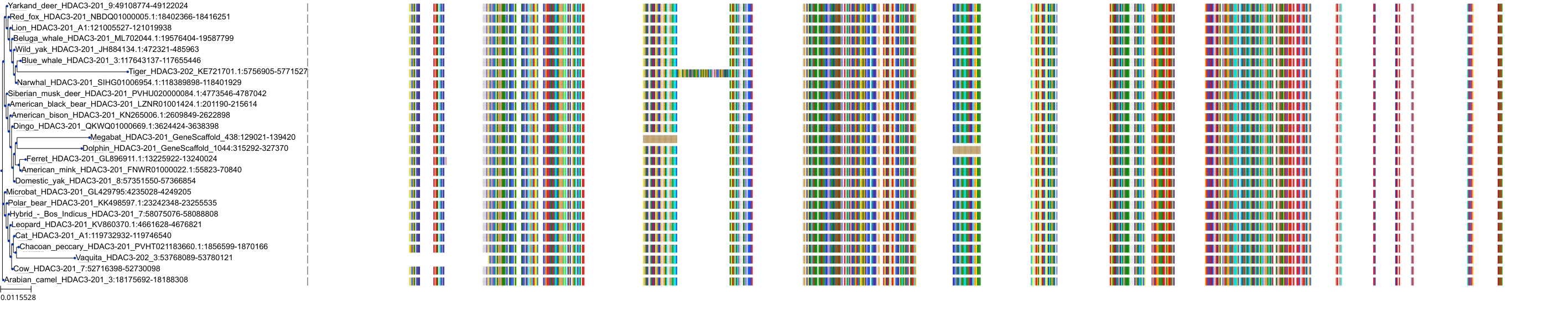
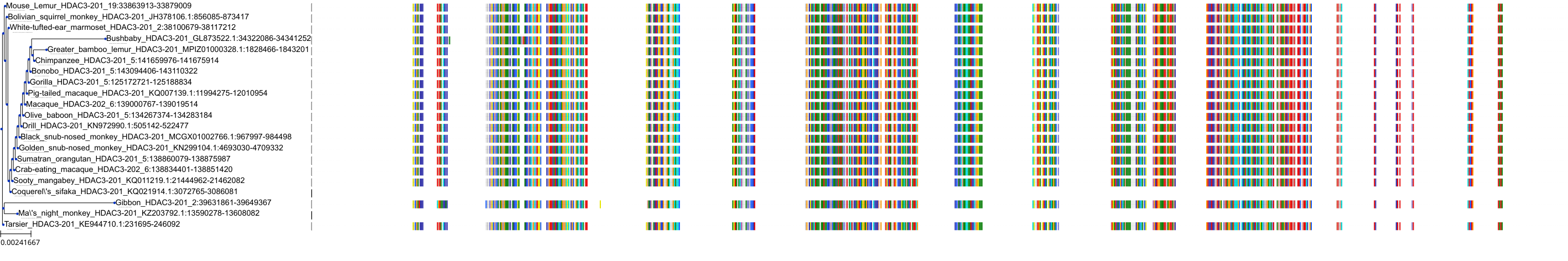
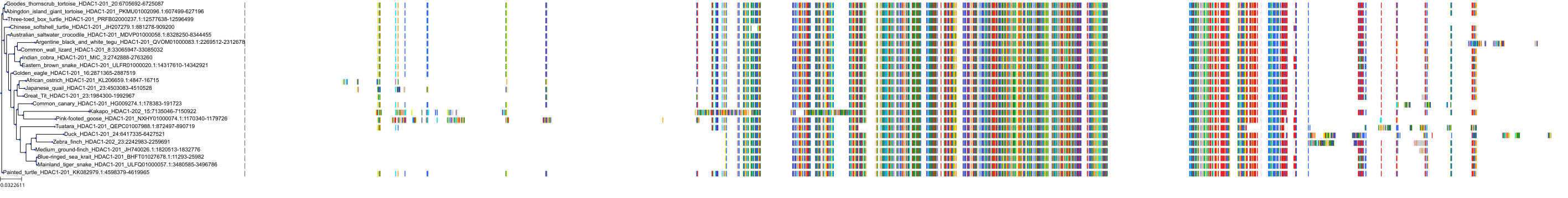
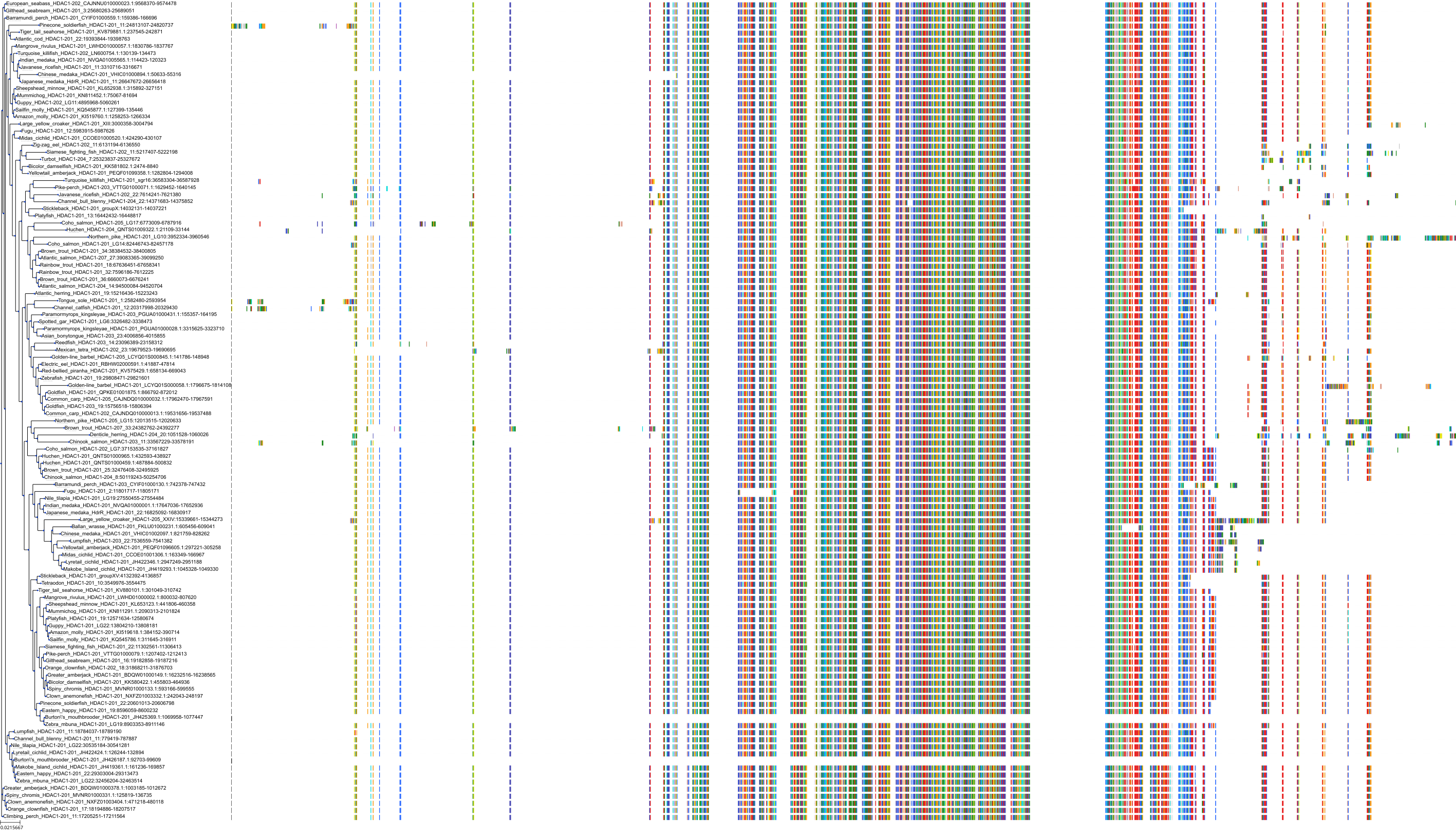
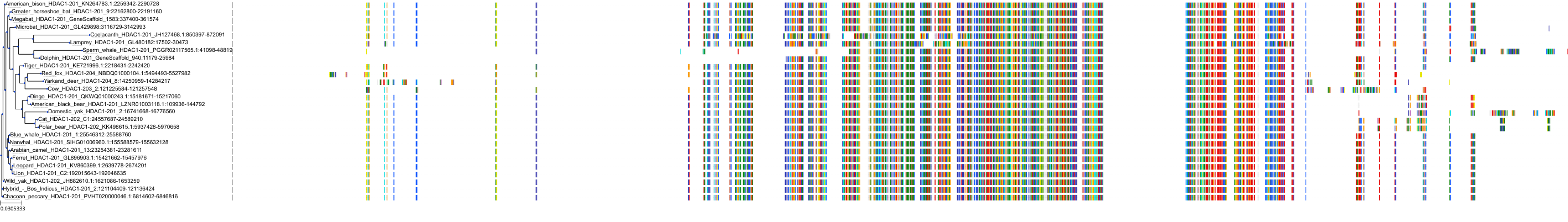
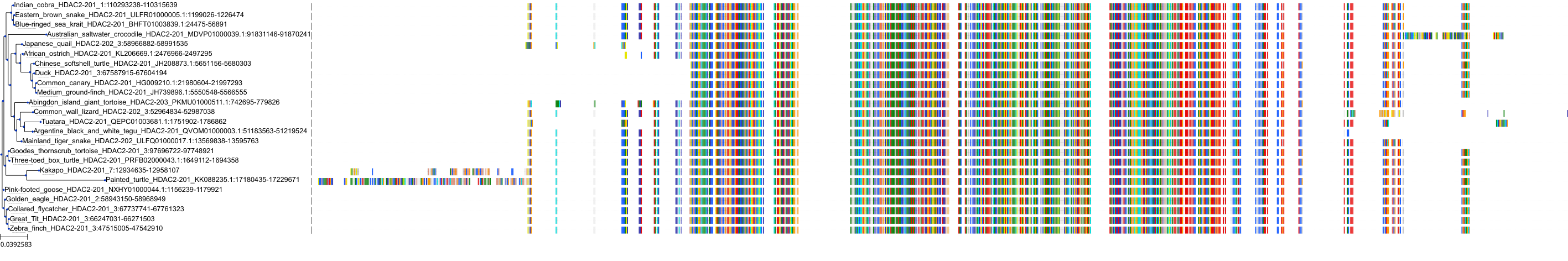
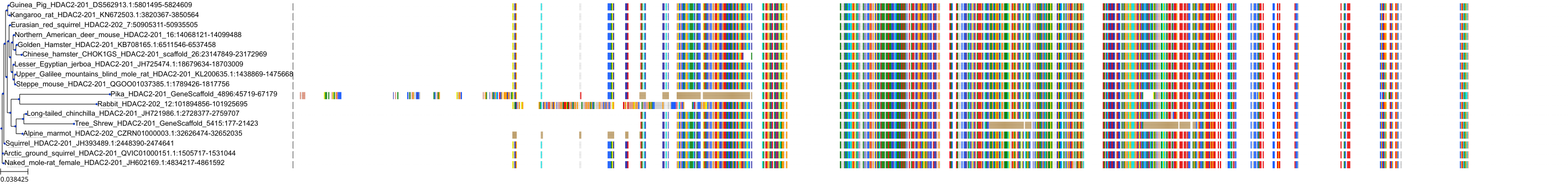
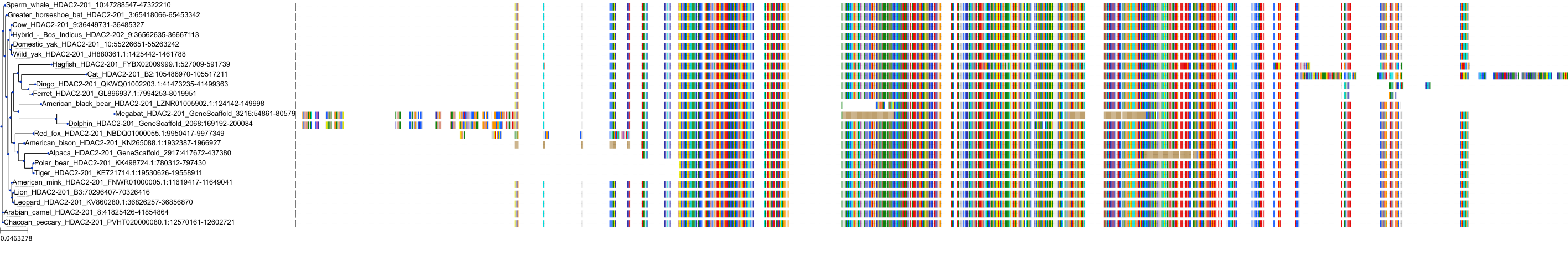
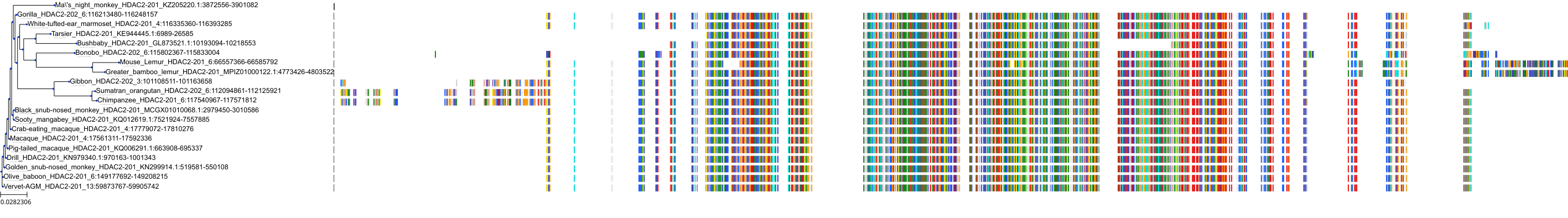
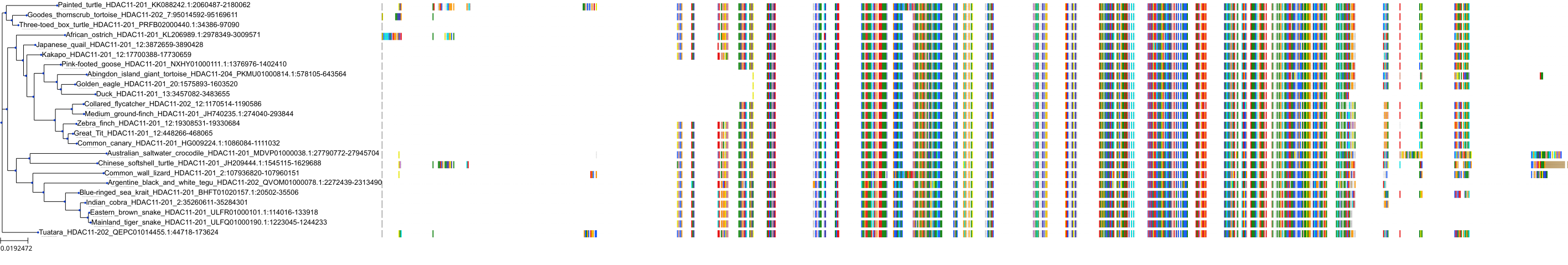
Target Conservation
|
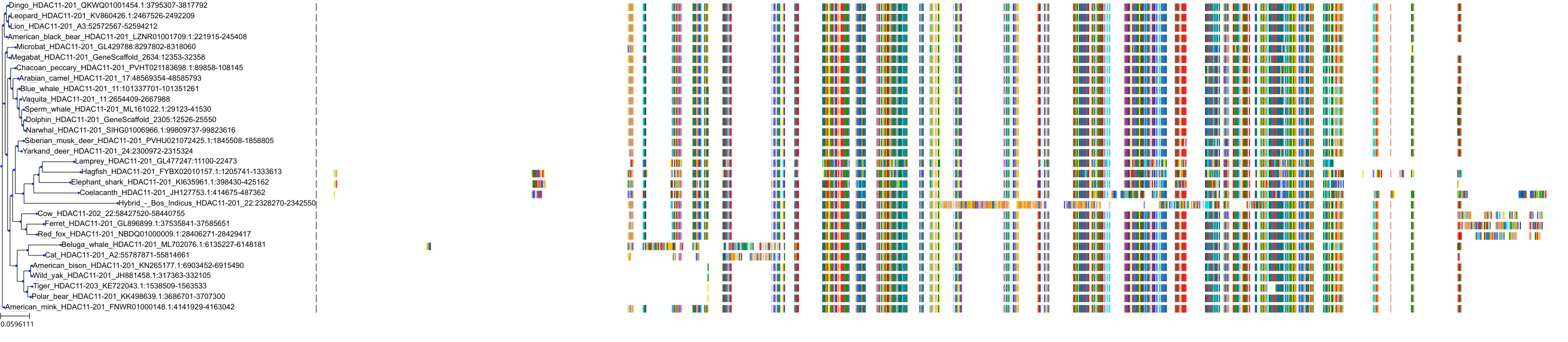
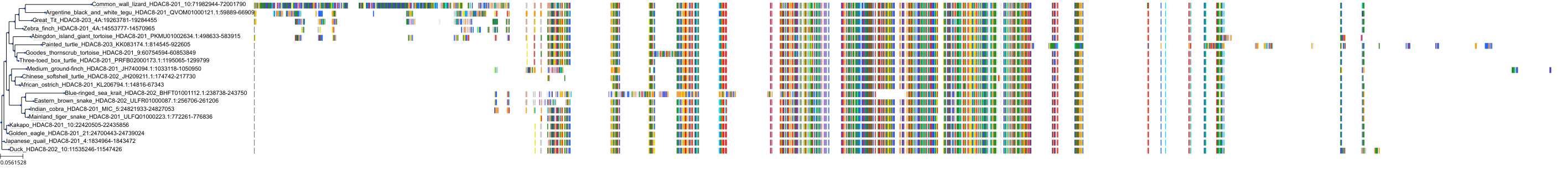
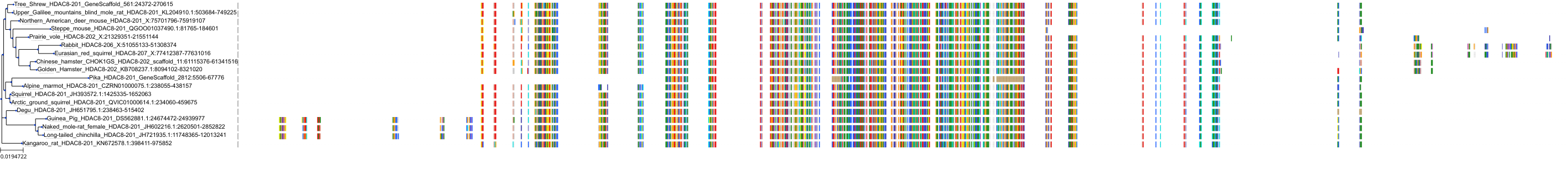
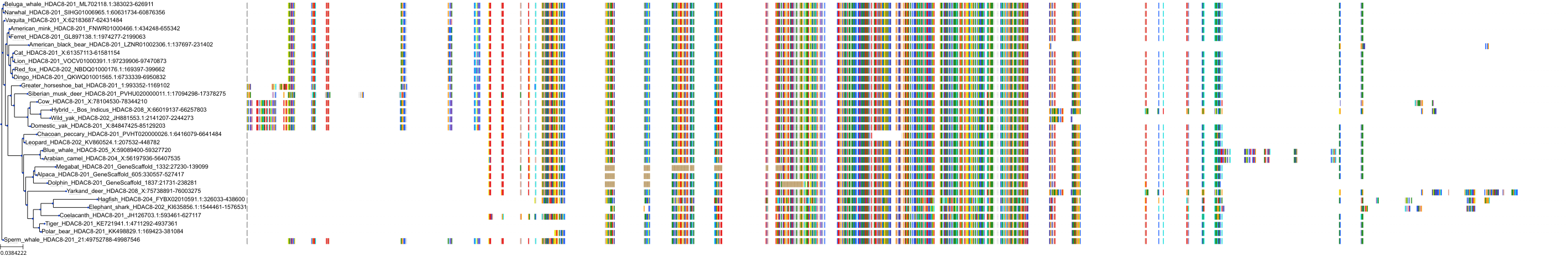
Protein: Histone deacetylase 3 Description: Histone deacetylase 3 Organism : Homo sapiens O15379 ENSG00000171720 |
||||
|
Protein: Histone deacetylase 1 Description: Histone deacetylase 1 Organism : Homo sapiens Q13547 ENSG00000116478 |
||||
|
Protein: Histone deacetylase 2 Description: Histone deacetylase 2 Organism : Homo sapiens Q92769 ENSG00000196591 |
||||
|
Protein: Histone deacetylase 11 Description: Histone deacetylase 11 Organism : Homo sapiens Q96DB2 ENSG00000163517 |
||||
|
Protein: Histone deacetylase 8 Description: Histone deacetylase 8 Organism : Homo sapiens Q9BY41 ENSG00000147099 |
||||
Related Entries
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 94525 |
| ChEMBL | CHEMBL272980 |
| DrugBank | DB11830 |
| FDA SRS | A6GWB8T96J |
| Guide to Pharmacology | 7008 |
| PubChem | 9865515 |
| SureChEMBL | SCHEMBL157027 |
| ZINC | ZINC000013986811 |
 Homo sapiens
Homo sapiens