| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| UNII | 41WC0AZ27V |
| EPA CompTox | DTXSID90242053 |
Structure
| InChI Key | IQOWHZHNGJXGHG-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C21H24FN3O2 |
| Molecular Weight | 369.44 |
| AlogP | 3.71 |
| Hydrogen Bond Acceptor | 4.0 |
| Number of Rotational Bond | 4.0 |
| Polar Surface Area | 45.67 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 2.0 |
| Heavy Atoms | 27.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Histamine H3 receptor antagonist | ANTAGONIST | PubMed PubMed PubMed |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Ion channel
Voltage-gated ion channel
Potassium channels
Voltage-gated potassium channel
|
- | - | - | - | 23 | |
|
Membrane receptor
Family A G protein-coupled receptor
Small molecule receptor (family A GPCR)
Monoamine receptor
Histamine receptor
|
- | - | 0.3802-3.548 | 1.4-23 | - |
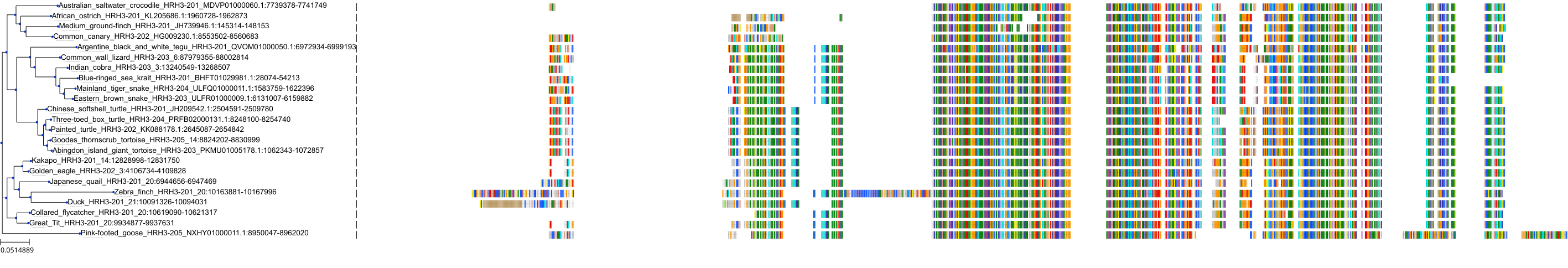
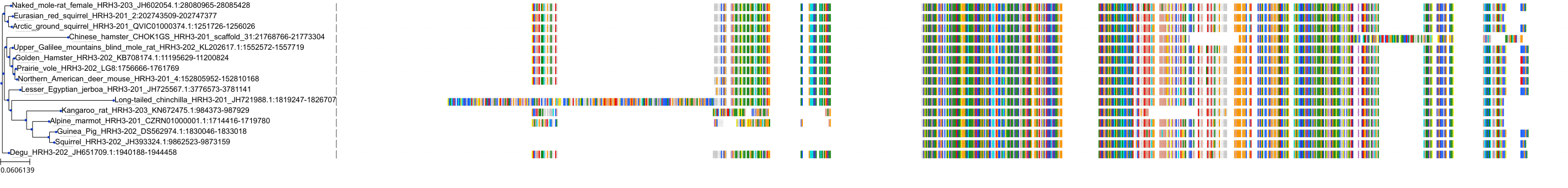
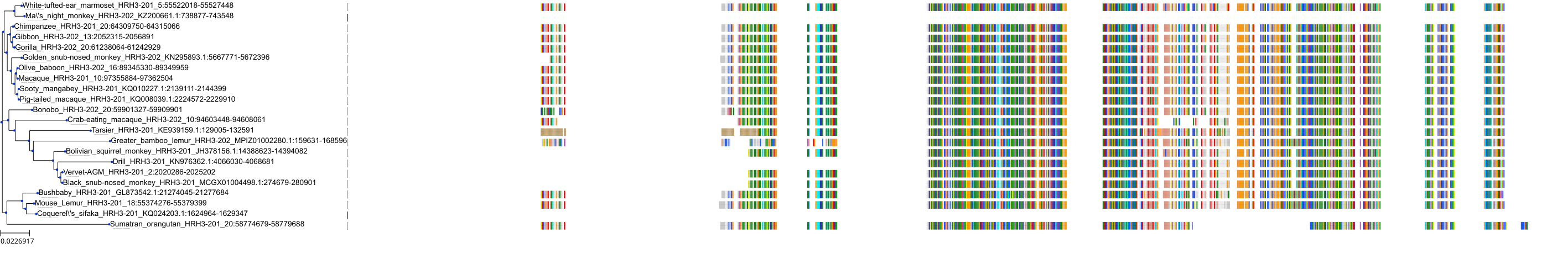
Target Conservation
|
Protein: Histamine H3 receptor Description: Histamine H3 receptor Organism : Homo sapiens Q9Y5N1 ENSG00000101180 |
||||
Related Entries
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL1171754 |
| DrugBank | DB12929 |
| FDA SRS | 41WC0AZ27V |
| PubChem | 24771368 |
| SureChEMBL | SCHEMBL524288 |
| ZINC | ZINC000053298428 |
 Homo sapiens
Homo sapiens
 Rattus norvegicus
Rattus norvegicus