| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| ATC | L01EX13 |
| UNII | 66D92MGC8M |
Structure
| InChI Key | GYQYAJJFPNQOOW-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C29H44N8O3 |
| Molecular Weight | 552.72 |
| AlogP | 2.7 |
| Hydrogen Bond Acceptor | 10.0 |
| Hydrogen Bond Donor | 3.0 |
| Number of Rotational Bond | 9.0 |
| Polar Surface Area | 121.11 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 2.0 |
| Heavy Atoms | 40.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Tyrosine-protein kinase receptor FLT3 inhibitor | INHIBITOR | Other PubMed FDA PubMed |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Enzyme
Kinase
Protein Kinase
TK protein kinase group
Tyrosine protein kinase Axl family
|
- | 0.73-11.3 | - | - | - | |
|
Enzyme
Kinase
Protein Kinase
TK protein kinase group
Tyrosine protein kinase PDGFR family
|
- | 0.29-0.29 | - | 2-10 | - | |
|
Transcription factor
|
- | 8.5 | - | - | - |
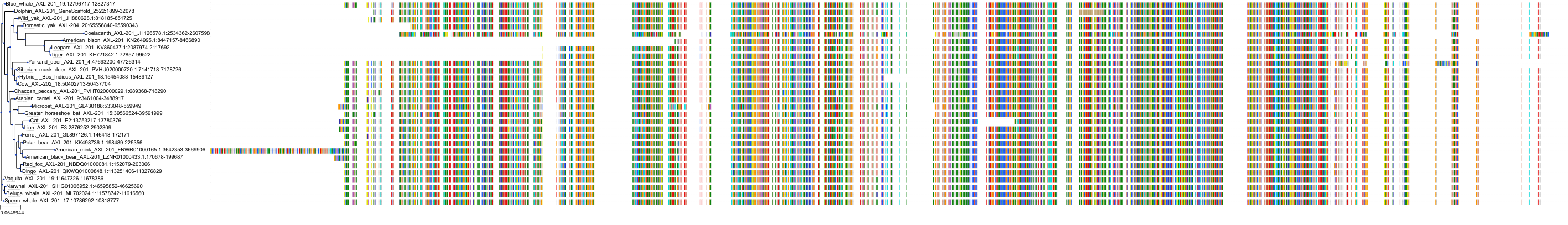
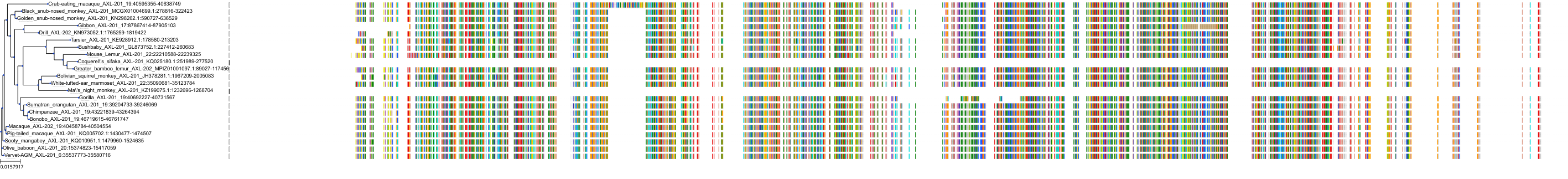
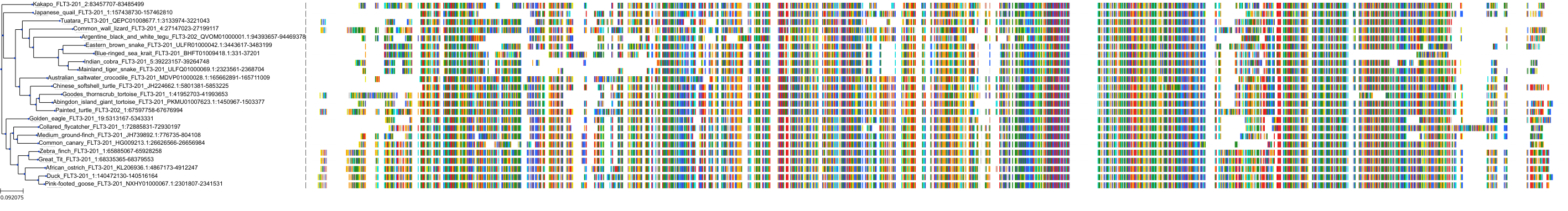
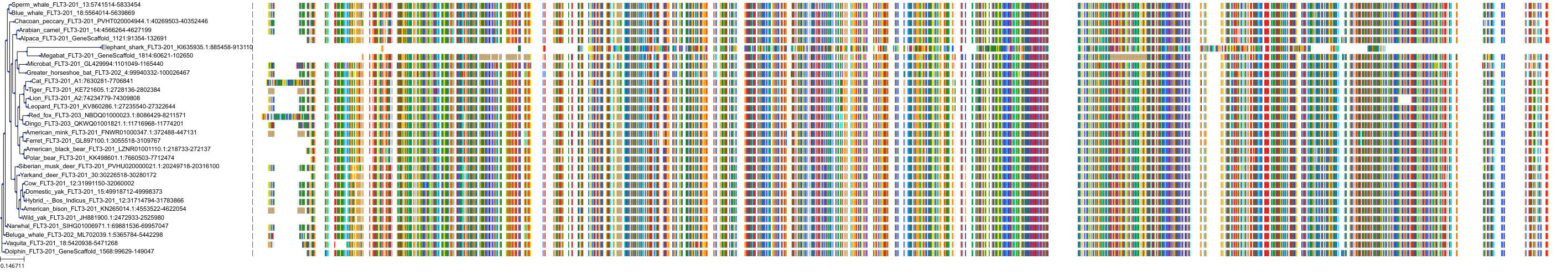
Target Conservation
|
Protein: Tyrosine-protein kinase receptor UFO Description: Tyrosine-protein kinase receptor UFO Organism : Homo sapiens P30530 ENSG00000167601 |
||||
|
Protein: Tyrosine-protein kinase receptor FLT3 Description: Receptor-type tyrosine-protein kinase FLT3 Organism : Homo sapiens P36888 ENSG00000122025 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 145372 |
| ChEMBL | CHEMBL3301622 |
| DrugBank | DB12141 |
| DrugCentral | 5306 |
| FDA SRS | 66D92MGC8M |
| Guide to Pharmacology | 8708 |
| PDB | C6F |
| PubChem | 49803313 |
| SureChEMBL | SCHEMBL282229 |
| ZINC | ZINC000113476229 |
 Homo sapiens
Homo sapiens
 Mus musculus
Mus musculus