| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| UNII | UI1U1MYH09 |
| EPA CompTox | DTXSID70189073 |
Structure
| InChI Key | WDPFJWLDPVQCAJ-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C36H38F4N4O2S |
| Molecular Weight | 666.79 |
| AlogP | 7.22 |
| Hydrogen Bond Acceptor | 6.0 |
| Number of Rotational Bond | 13.0 |
| Polar Surface Area | 58.44 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 4.0 |
| Heavy Atoms | 47.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| LDL-associated phospholipase A2 inhibitor | INHIBITOR | PubMed |
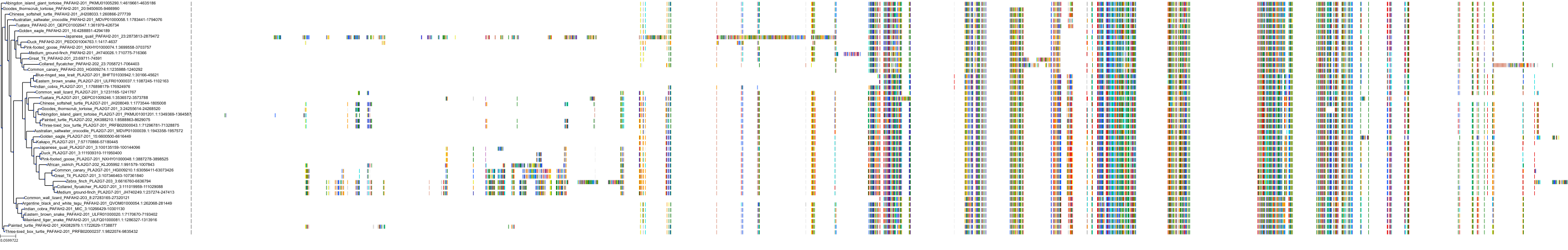
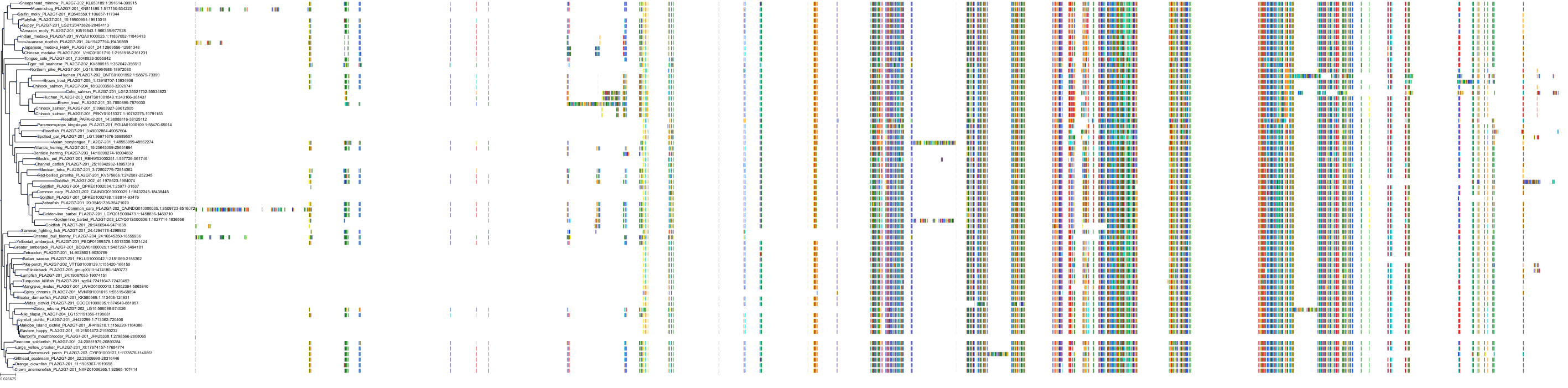
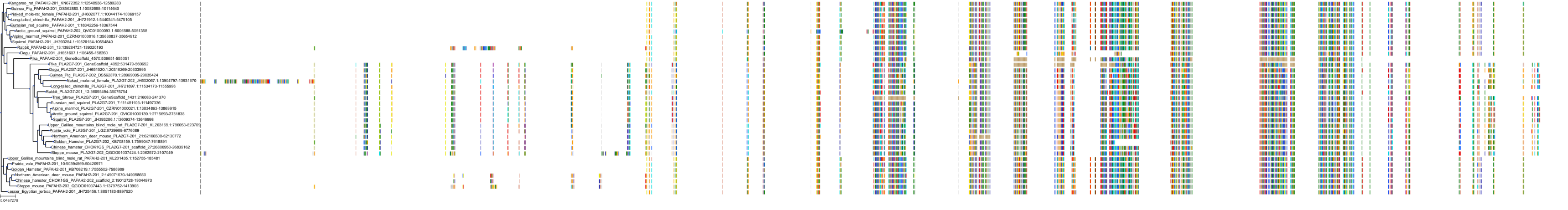
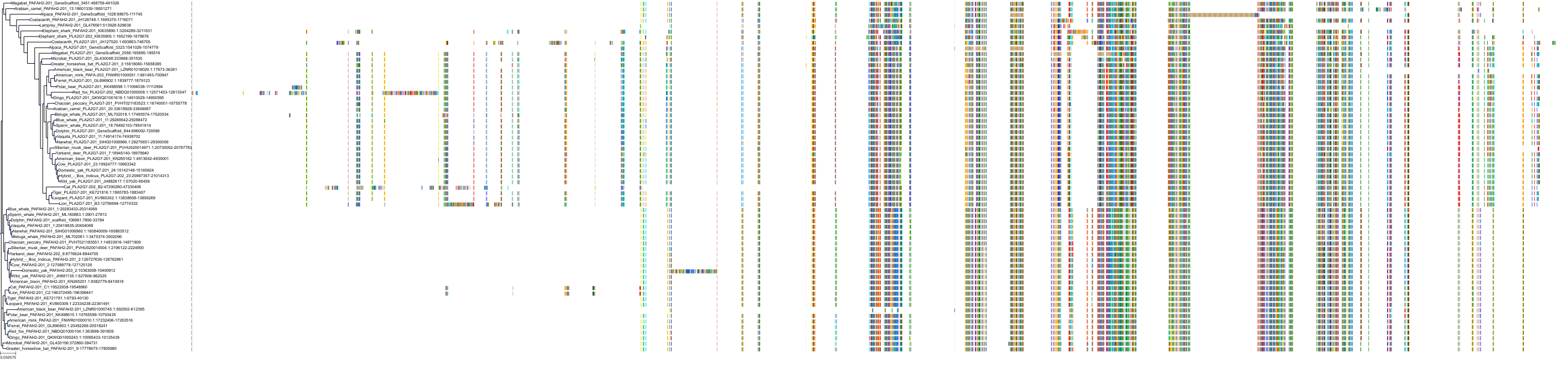
Target Conservation
|
Protein: LDL-associated phospholipase A2 Description: Platelet-activating factor acetylhydrolase Organism : Homo sapiens Q13093 ENSG00000146070 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL204021 |
| DrugBank | DB06311 |
| FDA SRS | UI1U1MYH09 |
| Guide to Pharmacology | 6696 |
| PDB | 5HV |
| PharmGKB | PA165884699 |
| PubChem | 9939609 |
| SureChEMBL | SCHEMBL2742709 |
| ZINC | ZINC000003842798 |
 Homo sapiens
Homo sapiens
 Mus musculus
Mus musculus
 Oryctolagus cuniculus
Oryctolagus cuniculus
 Rattus norvegicus
Rattus norvegicus