| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| ATC | C09CA06 |
| UNII | S8Q36MD2XX |
| EPA CompTox | DTXSID0022725 |
Structure
| InChI Key | HTQMVQVXFRQIKW-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C24H20N6O3 |
| Molecular Weight | 440.46 |
| AlogP | 4.03 |
| Hydrogen Bond Acceptor | 7.0 |
| Hydrogen Bond Donor | 2.0 |
| Number of Rotational Bond | 7.0 |
| Polar Surface Area | 118.81 |
| Molecular species | ACID |
| Aromatic Rings | 5.0 |
| Heavy Atoms | 33.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Type-1 angiotensin II receptor antagonist | ANTAGONIST | PubMed PubMed Other |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Membrane receptor
Family A G protein-coupled receptor
Peptide receptor (family A GPCR)
Short peptide receptor (family A GPCR)
Angiotensin receptor
|
- | 0.69-110 | 3.162-10 | 0.64-10.4 | 7-100 | |
|
Transporter
Electrochemical transporter
SLC superfamily of solute carriers
SLC21/SLCO family of organic anion transporting polypeptides
|
- | - | - | - | 28.4-52.1 | |
|
Transporter
Electrochemical transporter
SLC superfamily of solute carriers
SLC22 family of organic cation and anion transporters
|
- | - | - | - | 6.3 | |
|
Unclassified protein
|
- | - | - | - | 98-100 |
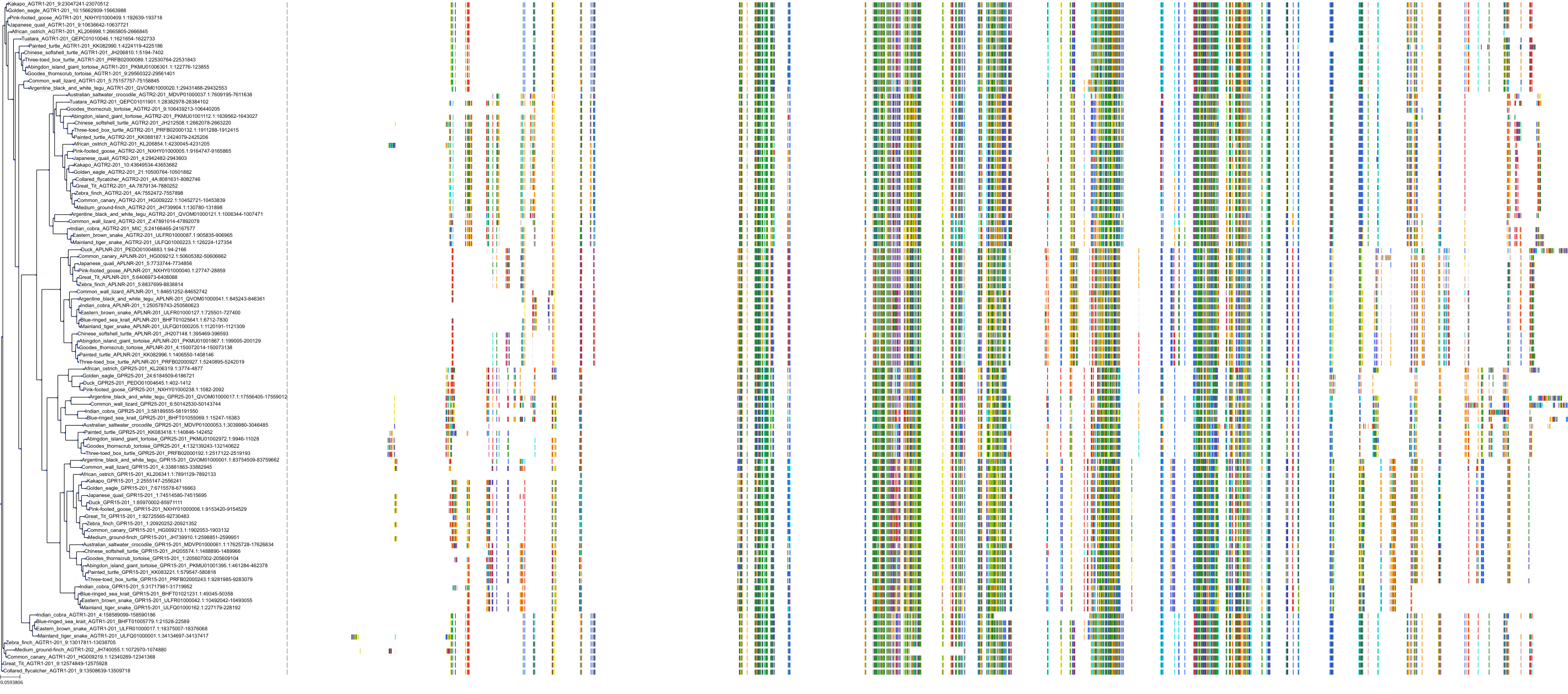
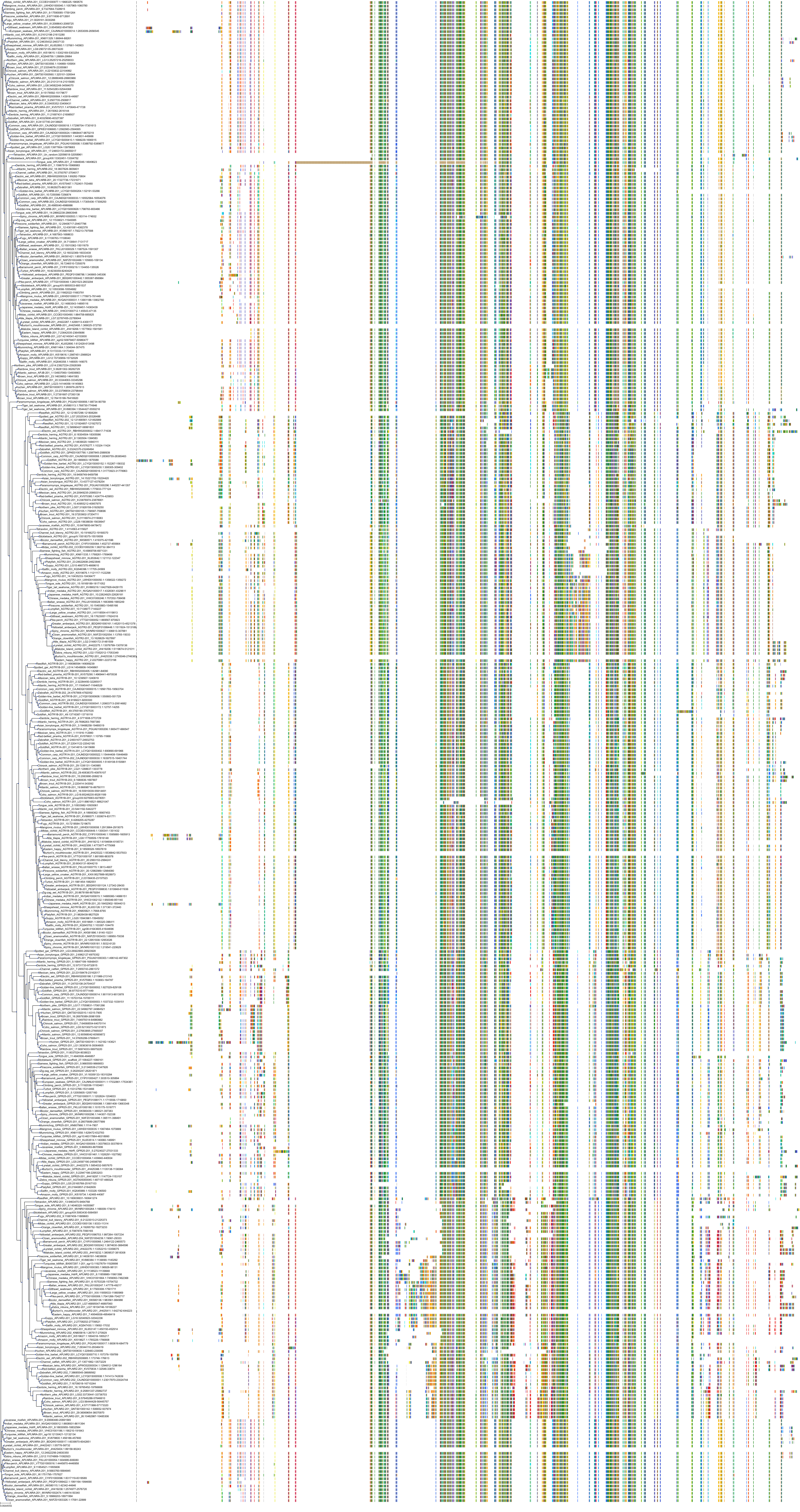
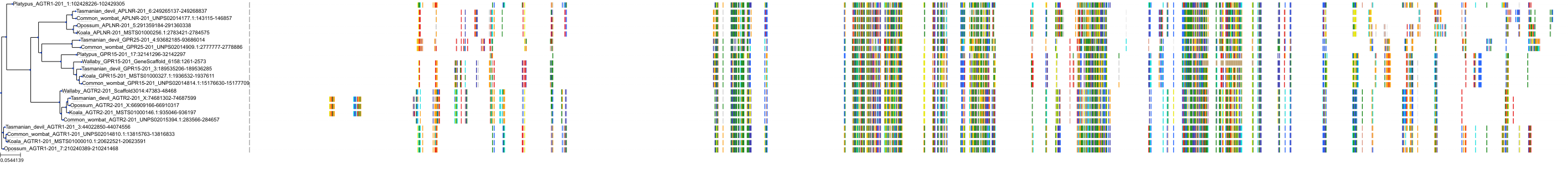
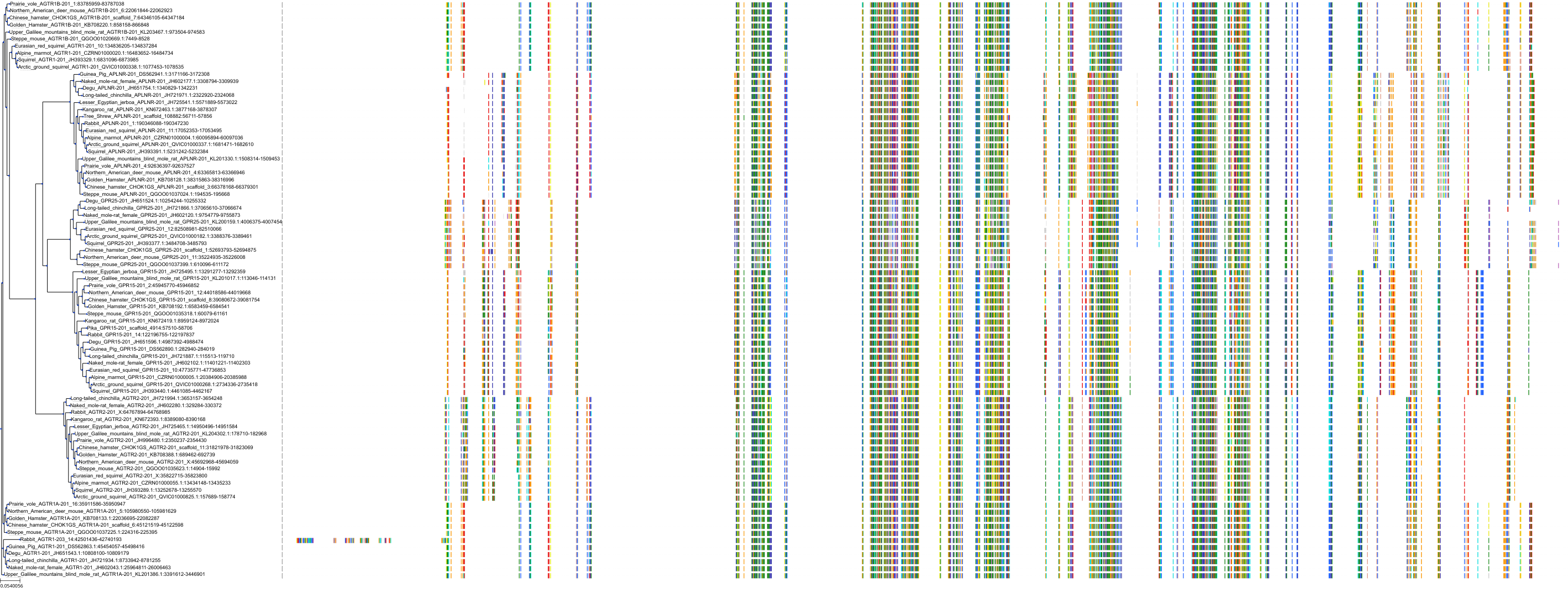
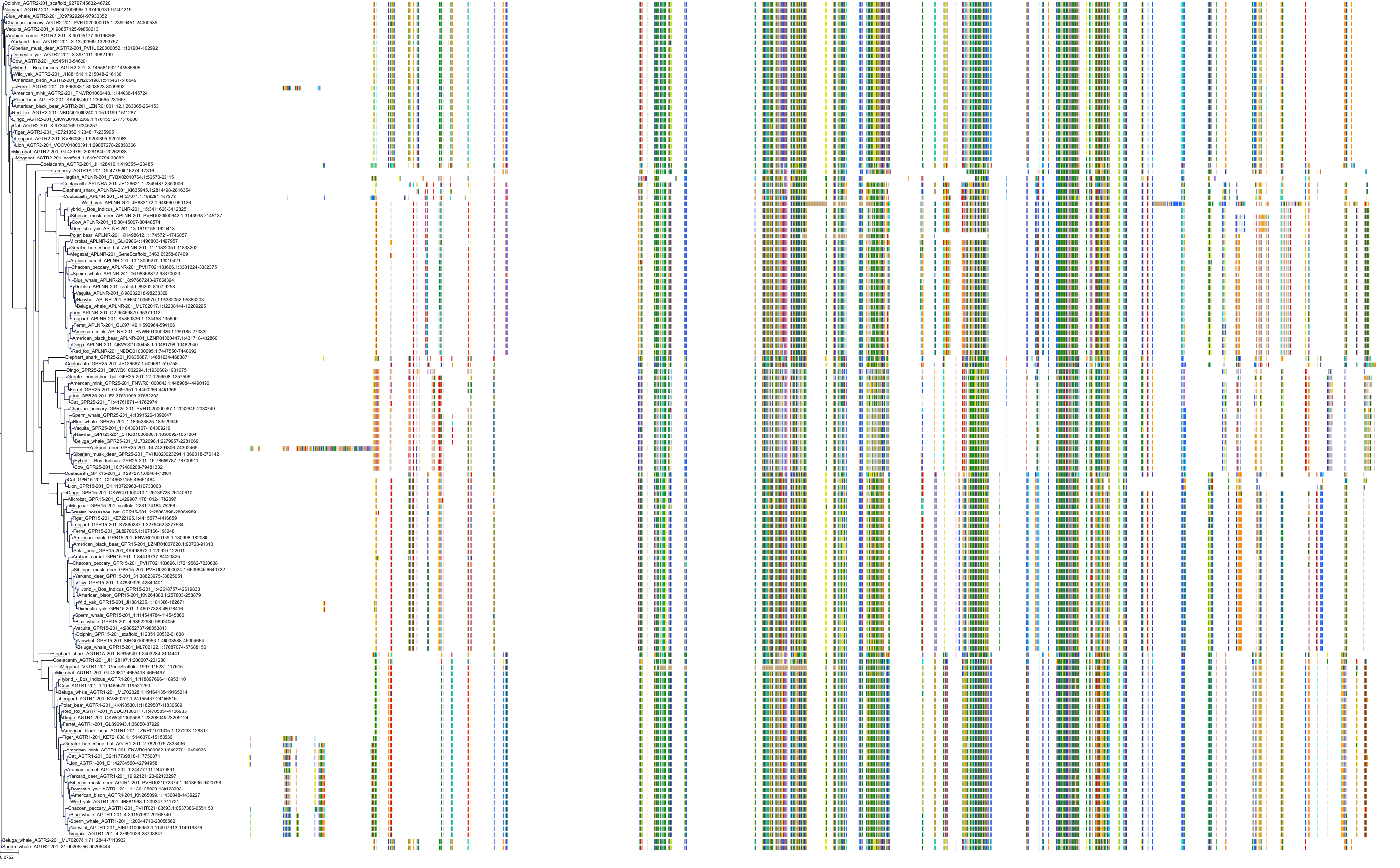
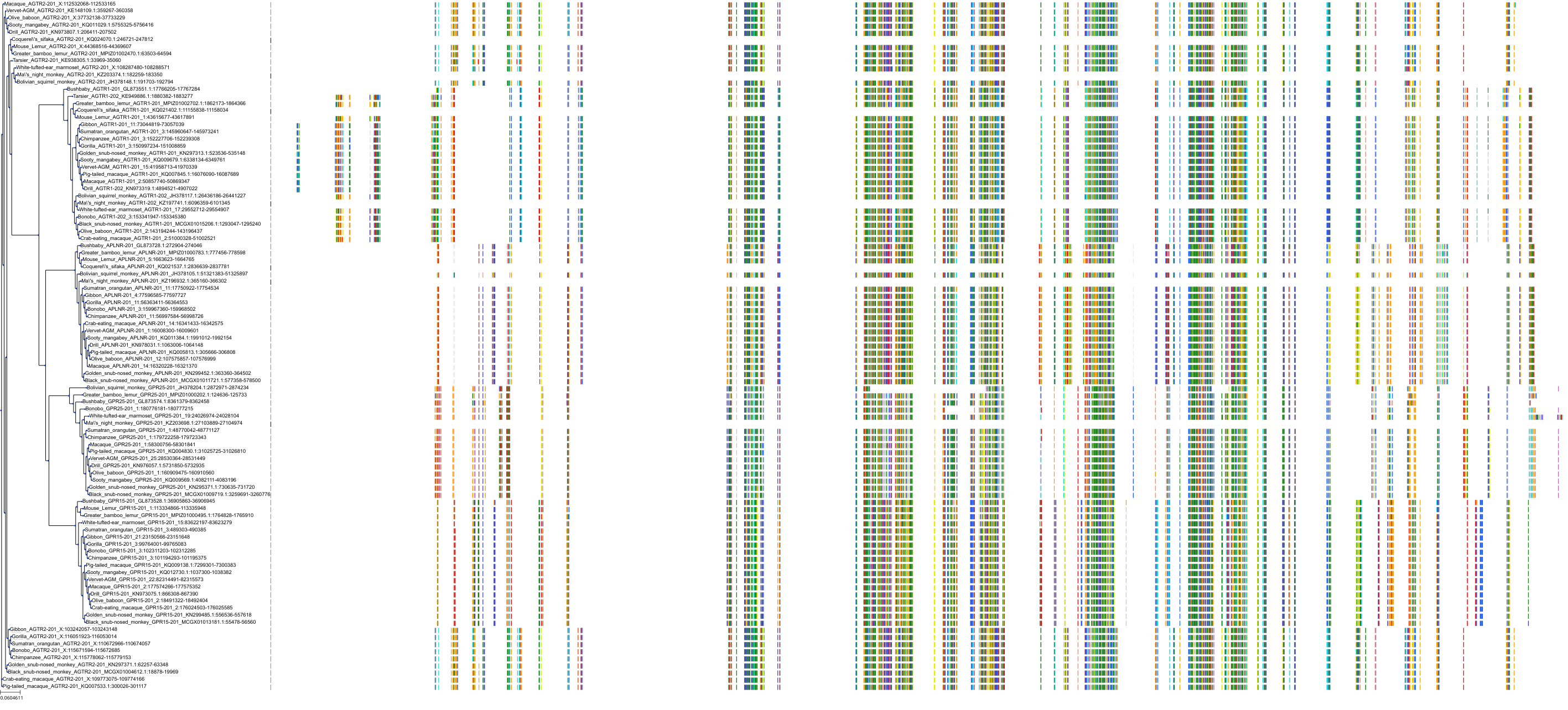
Target Conservation
|
Protein: Type-1 angiotensin II receptor Description: Type-1 angiotensin II receptor Organism : Homo sapiens P30556 ENSG00000144891 |
||||
Related Entries
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 3347 |
| ChEMBL | CHEMBL1016 |
| DrugBank | DB13919 |
| FDA SRS | S8Q36MD2XX |
| Human Metabolome Database | HMDB0014934 |
| Guide to Pharmacology | 587 |
| PharmGKB | PA448765 |
| PubChem | 2541 |
| SureChEMBL | SCHEMBL3938 |
| ZINC | ZINC000003782818 |
 Bos taurus
Bos taurus
 Escherichia coli
Escherichia coli
 Homo sapiens
Homo sapiens
 Oryctolagus cuniculus
Oryctolagus cuniculus
 Rattus norvegicus
Rattus norvegicus