Structure
| InChI Key | XJFMHMFFBSOEPR-DNZQAUTHSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C17H25N7O4 |
| Molecular Weight | 391.43 |
| Hydrogen Bond Acceptor | 11.0 |
| Hydrogen Bond Donor | 5.0 |
| Number of Rotational Bond | 5.0 |
| Polar Surface Area | 163.93 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 2.0 |
| Heavy Atoms | 28.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Adenosine A2a receptor agonist | AGONIST | PubMed |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Membrane receptor
Family A G protein-coupled receptor
Small molecule receptor (family A GPCR)
Nucleotide-like receptor (family A GPCR)
Adenosine receptor
|
0.257 | - | - | 25.5-903 | 17 |
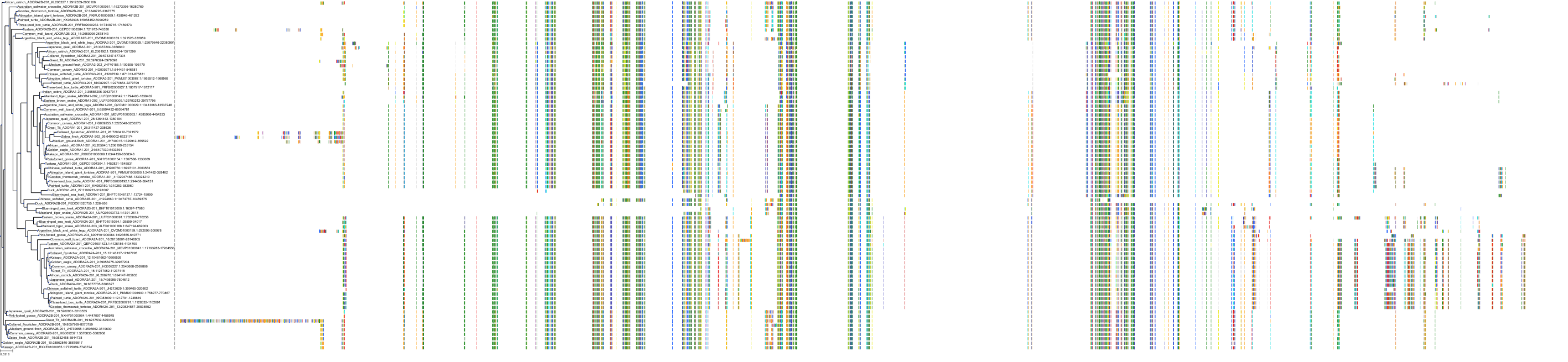
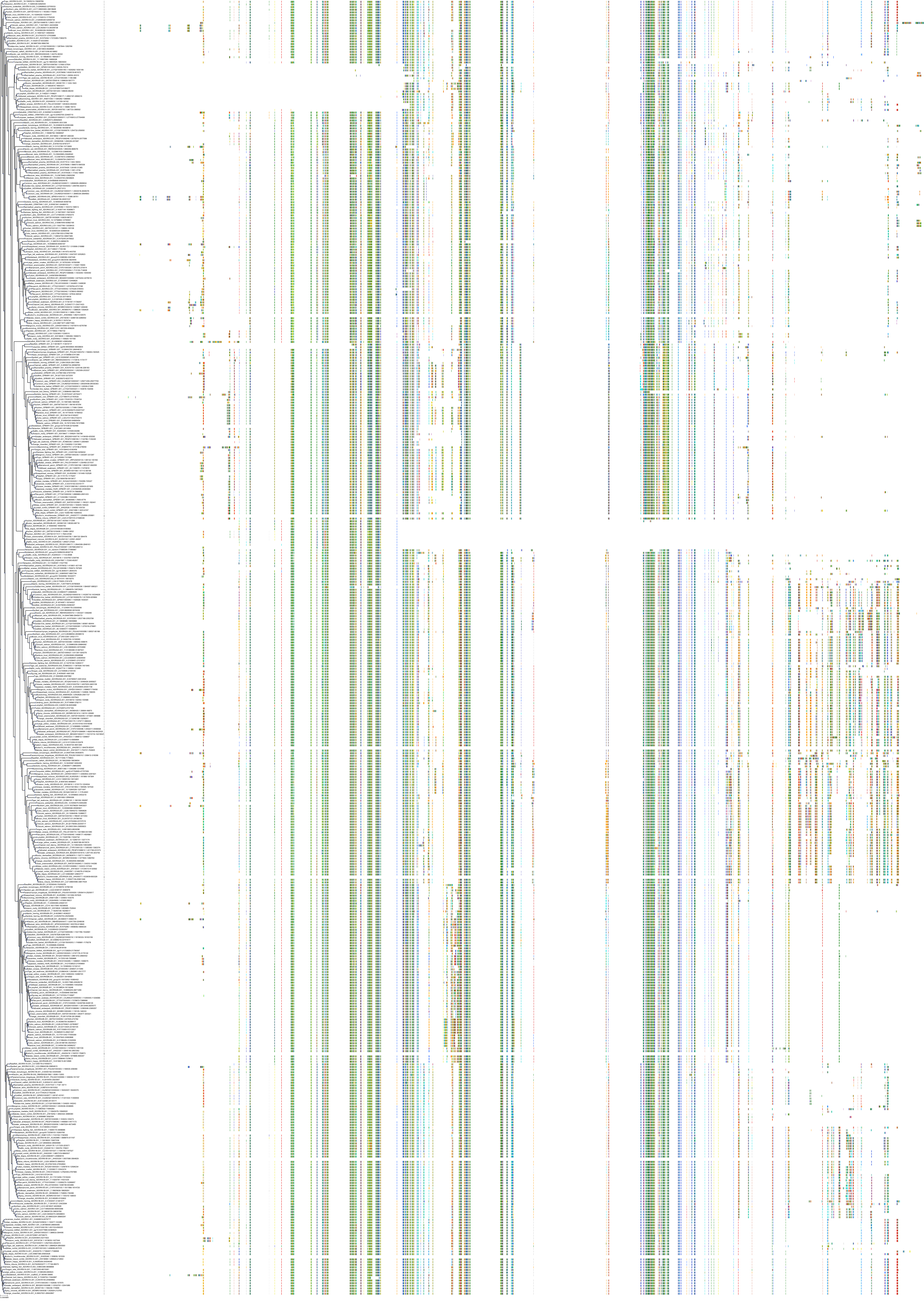
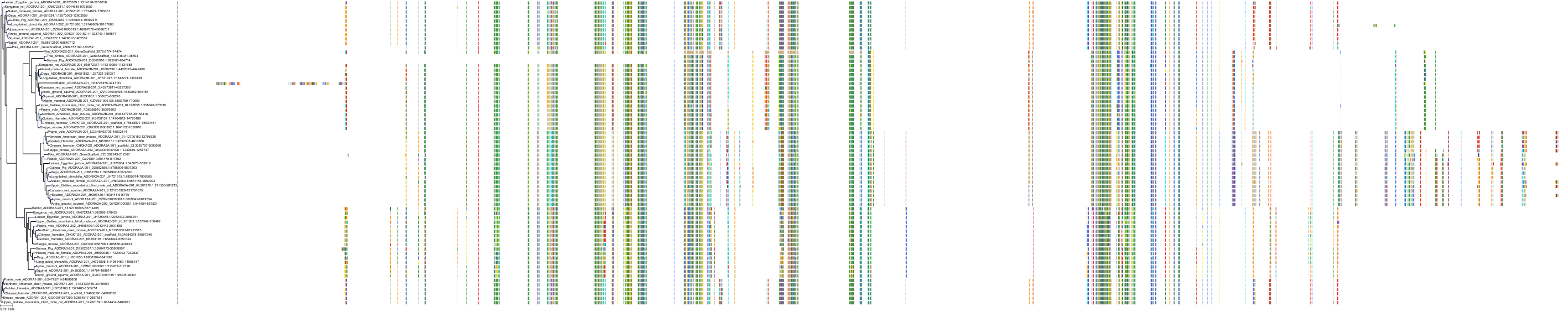
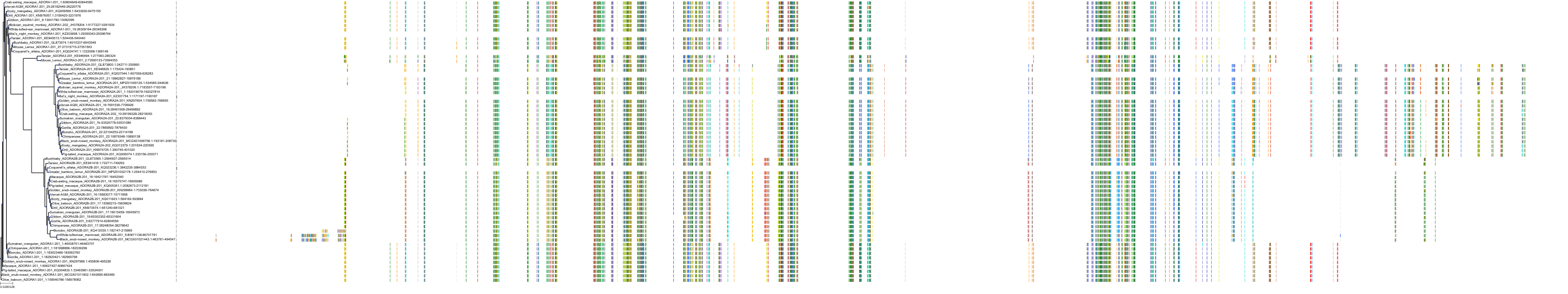
Target Conservation
|
Protein: Adenosine A2a receptor Description: Adenosine receptor A2a Organism : Homo sapiens P29274 ENSG00000128271 |
||||
Related Entries
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL1950554 |
| DrugBank | DB04853 |
| FDA SRS | LJA4M1L5LG |
| Guide to Pharmacology | 5595 |
| PubChem | 9576912 |
| SureChEMBL | SCHEMBL724955 |
 Cavia porcellus
Cavia porcellus
 Homo sapiens
Homo sapiens
 Rattus norvegicus
Rattus norvegicus