Structure
| InChI Key | ZGBAJMQHJDFTQJ-DEOSSOPVSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C30H31F3N8O |
| Molecular Weight | 576.63 |
| AlogP | 5.39 |
| Hydrogen Bond Acceptor | 8.0 |
| Hydrogen Bond Donor | 2.0 |
| Number of Rotational Bond | 8.0 |
| Polar Surface Area | 99.17 |
| Molecular species | BASE |
| Aromatic Rings | 4.0 |
| Heavy Atoms | 42.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Tyrosine-protein kinase ABL inhibitor | INHIBITOR | PubMed |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Enzyme
Kinase
Protein Kinase
TK protein kinase group
Tyrosine protein kinase Abl family
|
- | 26 | - | - | - | |
|
Enzyme
Kinase
Protein Kinase
TK protein kinase group
Tyrosine protein kinase Src family
|
- | 11 | - | - | - |
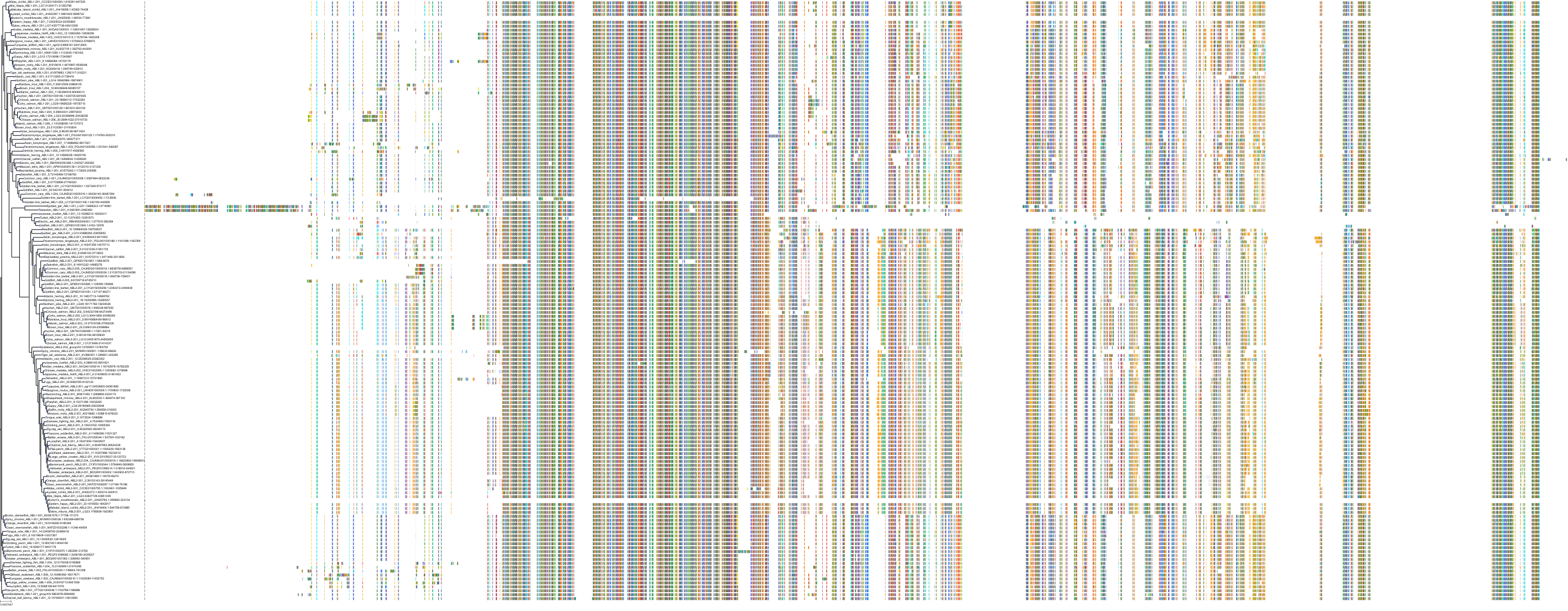
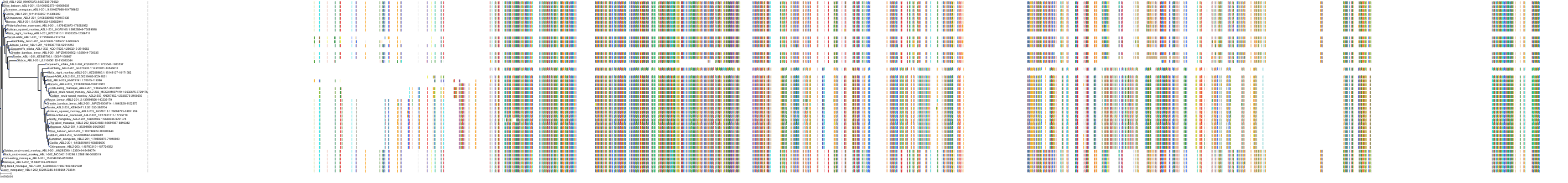
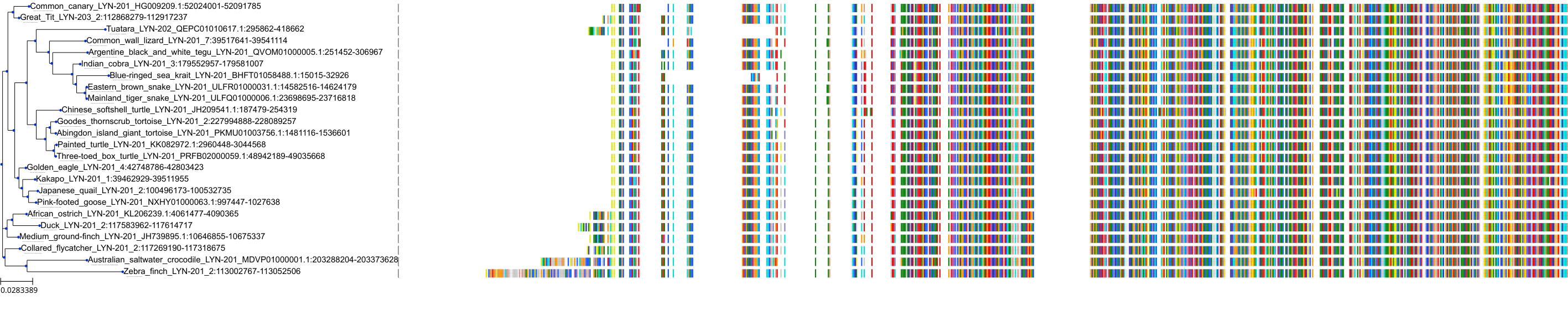
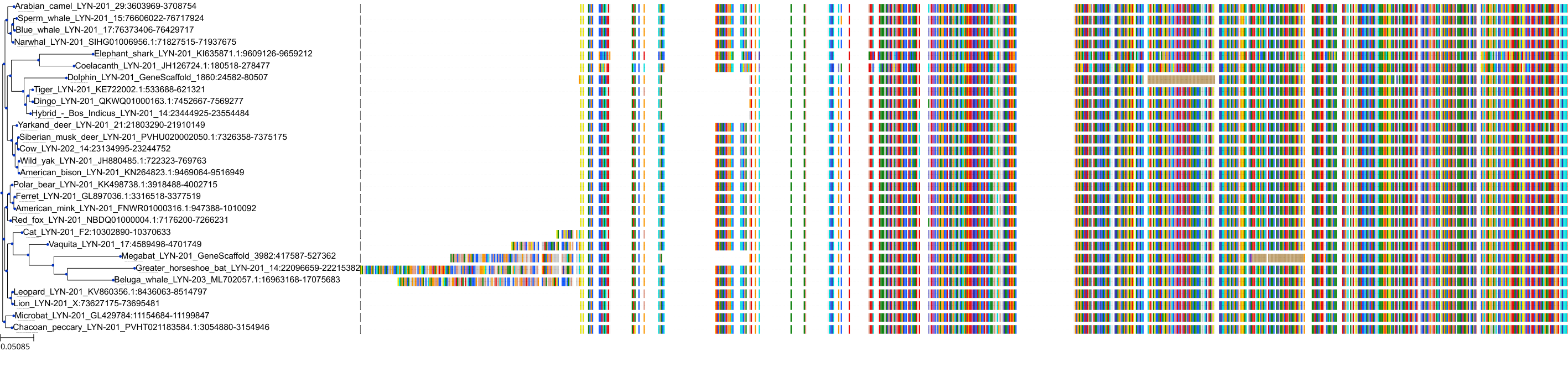
Target Conservation
|
Protein: Tyrosine-protein kinase ABL Description: Tyrosine-protein kinase ABL1 Organism : Homo sapiens P00519 ENSG00000097007 |
||||
|
Protein: Tyrosine-protein kinase Lyn Description: Tyrosine-protein kinase Lyn Organism : Homo sapiens P07948 ENSG00000254087 |
||||
Related Entries
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL206834 |
| DrugBank | DB11851 |
| FDA SRS | NVW4Z03I9B |
| Human Metabolome Database | HMDB0240206 |
| Guide to Pharmacology | 7906 |
| PDB | 406 |
| PubChem | 11387605 |
| SureChEMBL | SCHEMBL2684766 |
| ZINC | ZINC000022940637 |
 Homo sapiens
Homo sapiens