| Trade Names | |
| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| ATC | L01XX08 |
| UNII | 395575MZO7 |
| EPA CompTox | DTXSID2023436 |
Structure
| InChI Key | FPVKHBSQESCIEP-JQCXWYLXSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C11H16N4O4 |
| Molecular Weight | 268.27 |
| AlogP | -1.18 |
| Hydrogen Bond Acceptor | 8.0 |
| Hydrogen Bond Donor | 4.0 |
| Number of Rotational Bond | 2.0 |
| Polar Surface Area | 112.13 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 1.0 |
| Heavy Atoms | 19.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Adenosine deaminase inhibitor | INHIBITOR | DailyMed |
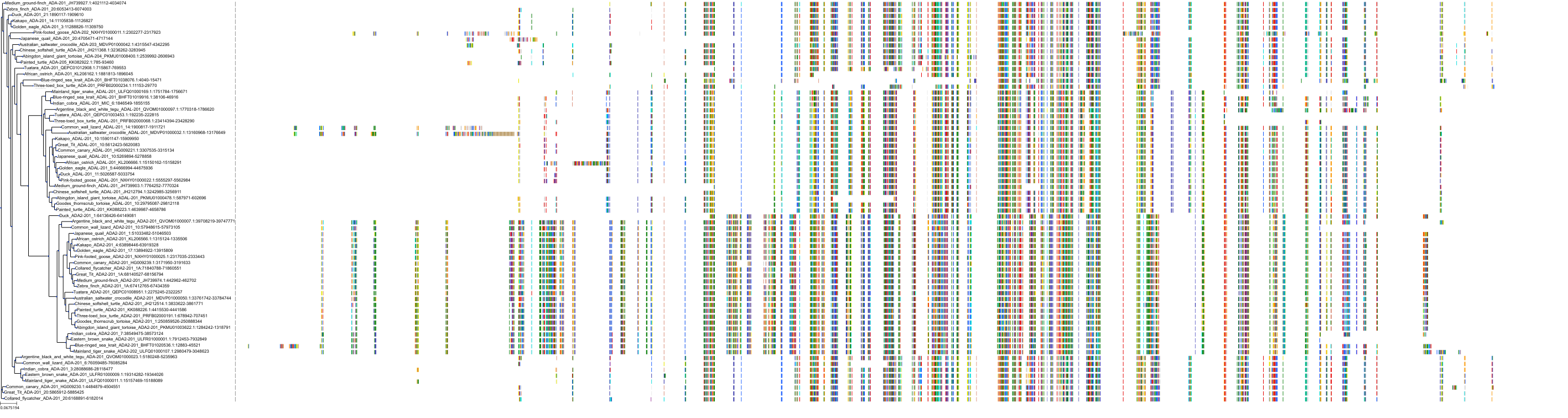
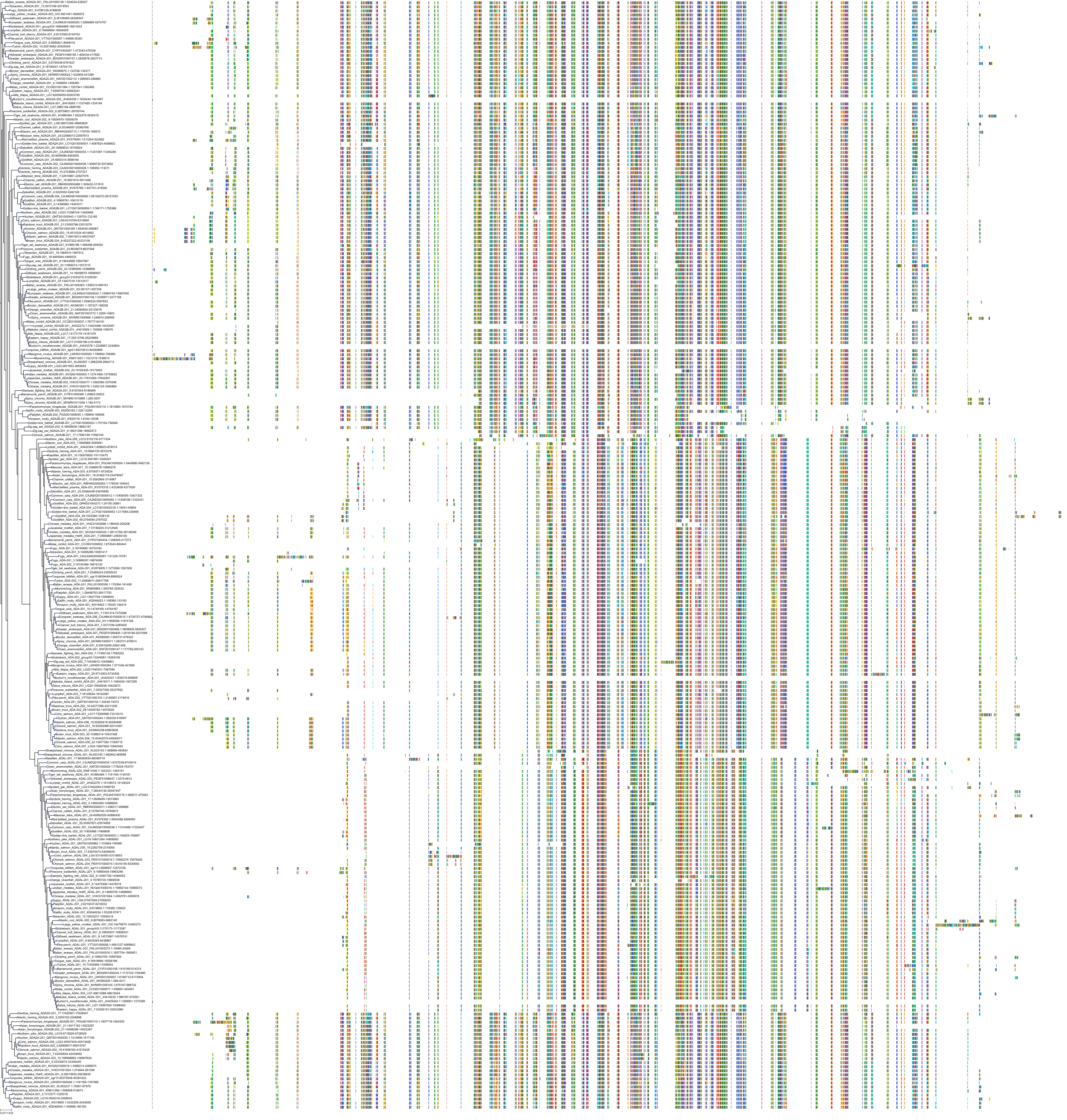
Target Conservation
|
Protein: Adenosine deaminase Description: Adenosine deaminase Organism : Homo sapiens P00813 ENSG00000196839 |
||||
Related Entries
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL1580 |
| DrugBank | DB00552 |
| DrugCentral | 2098 |
| FDA SRS | 395575MZO7 |
| Human Metabolome Database | HMDB0014692 |
| Guide to Pharmacology | 4805 |
| KEGG | C02267 |
| PDB | DCF |
| PharmGKB | PA450863 |
| PubChem | 439693 |
| SureChEMBL | SCHEMBL2817 |
| ZINC | ZINC000003806262 |
 Homo sapiens
Homo sapiens
 Mus musculus
Mus musculus
 Plasmodium falciparum
Plasmodium falciparum