| Trade Names | |
| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| ATC | A10BH05 |
| UNII | 3X29ZEJ4R2 |
Structure
| InChI Key | LTXREWYXXSTFRX-QGZVFWFLSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C25H28N8O2 |
| Molecular Weight | 472.55 |
| AlogP | 1.15 |
| Hydrogen Bond Acceptor | 10.0 |
| Hydrogen Bond Donor | 1.0 |
| Number of Rotational Bond | 4.0 |
| Polar Surface Area | 116.86 |
| Molecular species | BASE |
| Aromatic Rings | 4.0 |
| Heavy Atoms | 35.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Dipeptidyl peptidase IV inhibitor | INHIBITOR | DailyMed |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Enzyme
Protease
Serine protease
Serine protease SC clan
Serine protease S9B subfamily
|
- | 0.1-370 | 0.0066-5.3 | - | 96.24 | |
|
Ion channel
Voltage-gated ion channel
Potassium channels
Voltage-gated potassium channel
|
- | - | - | - | 97 | |
|
Membrane receptor
Family A G protein-coupled receptor
Small molecule receptor (family A GPCR)
Monoamine receptor
Acetylcholine receptor
|
- | 295-300 | - | - | - | |
|
Transporter
Electrochemical transporter
SLC superfamily of solute carriers
SLC21/SLCO family of organic anion transporting polypeptides
|
- | - | - | - | 3.39-35.5 | |
|
Transporter
Electrochemical transporter
SLC superfamily of solute carriers
SLC22 family of organic cation and anion transporters
|
- | - | - | - | 0-19.4 | |
|
Transporter
Primary active transporter
ATP-binding cassette
ABCG subfamily
|
- | - | - | - | 24.7 |
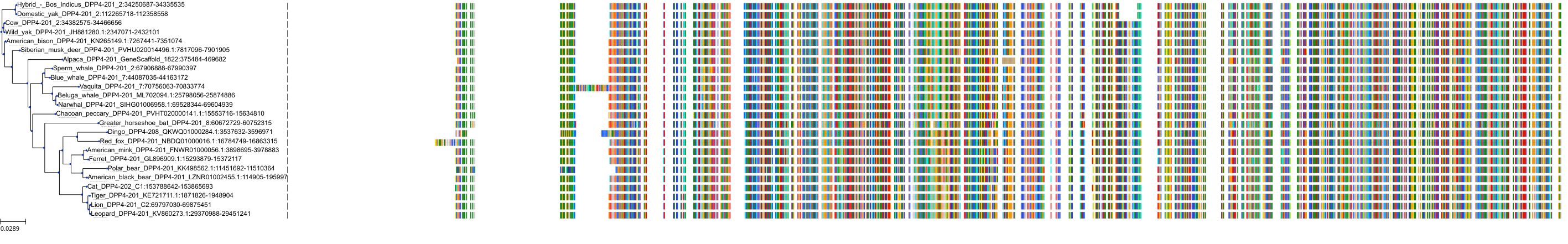
Target Conservation
|
Protein: Dipeptidyl peptidase IV Description: Dipeptidyl peptidase 4 Organism : Homo sapiens P27487 ENSG00000197635 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 68610 |
| ChEMBL | CHEMBL237500 |
| DrugBank | DB08882 |
| DrugCentral | 4175 |
| FDA SRS | 3X29ZEJ4R2 |
| Guide to Pharmacology | 6318 |
| PDB | 356 |
| PubChem | 10096344 |
| SureChEMBL | SCHEMBL160188 |
| ZINC | ZINC000003820029 |
 Homo sapiens
Homo sapiens
 Mus musculus
Mus musculus