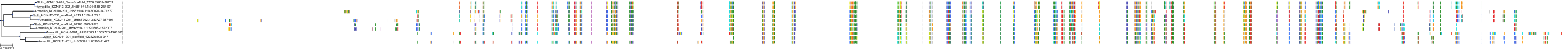| Trade Names | |
| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| UNII | SX6K58TVWC |
| EPA CompTox | DTXSID0037237 |
Structure
| InChI Key | ZNNLBTZKUZBEKO-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C23H28ClN3O5S |
| Molecular Weight | 494.01 |
| AlogP | 3.64 |
| Hydrogen Bond Acceptor | 5.0 |
| Hydrogen Bond Donor | 3.0 |
| Number of Rotational Bond | 8.0 |
| Polar Surface Area | 113.6 |
| Molecular species | ACID |
| Aromatic Rings | 2.0 |
| Heavy Atoms | 33.0 |
Pharmacology
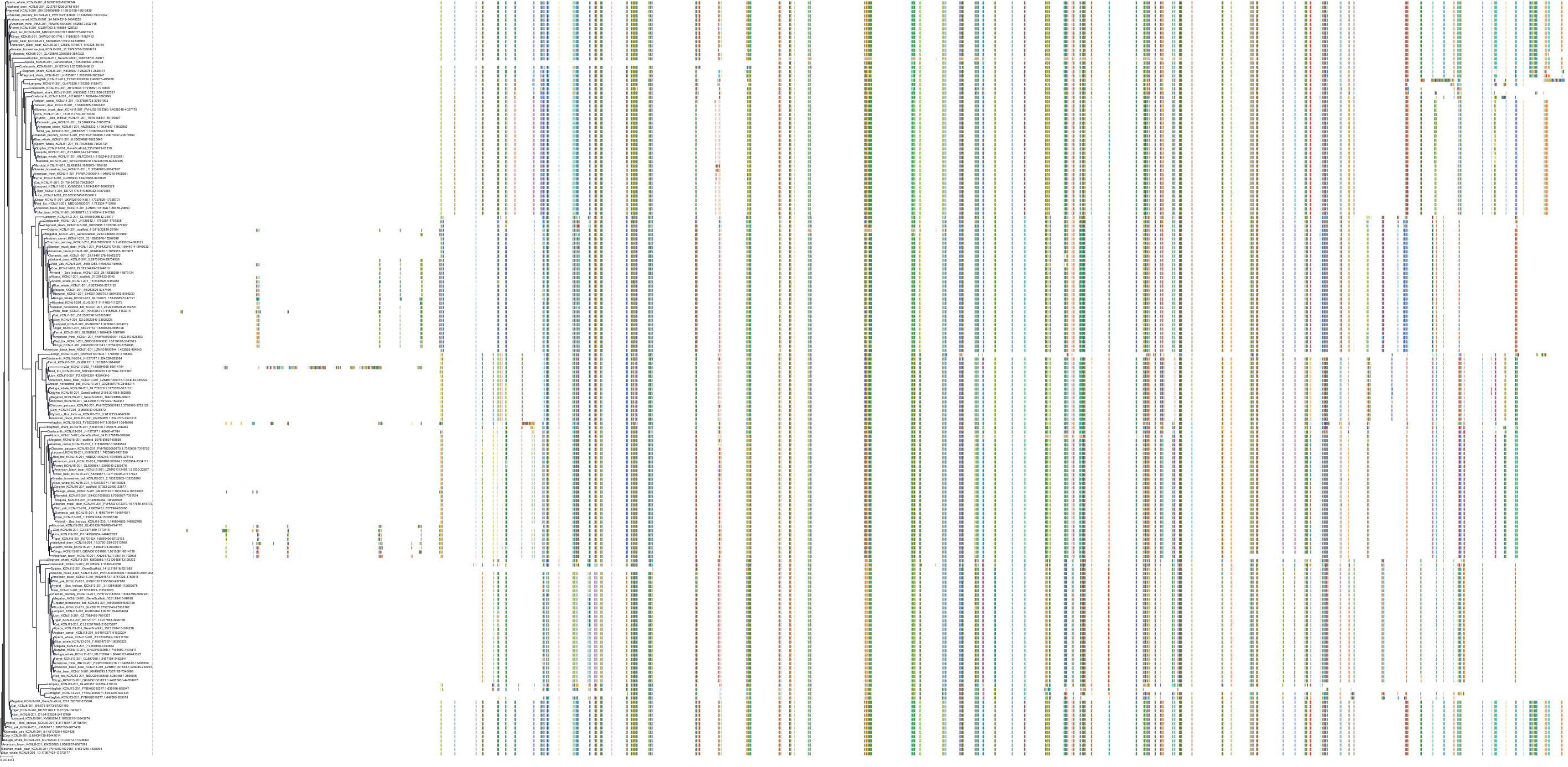
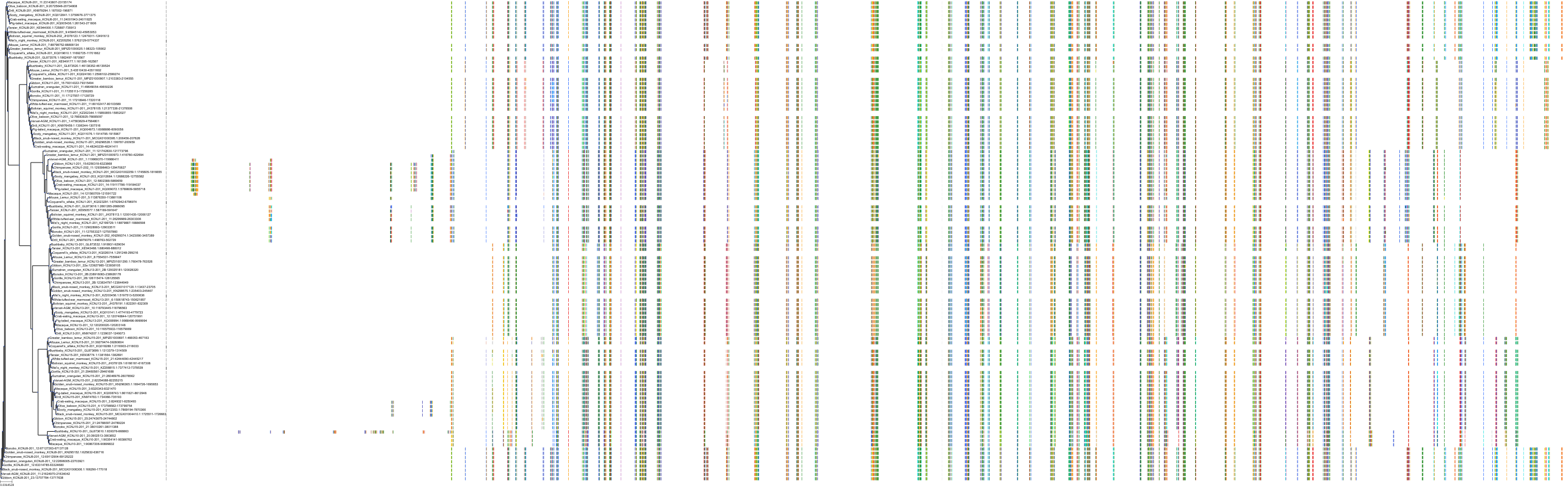
Target Conservation
|
Protein: Sulfonylurea receptor 1, Kir6.2 Description: ATP-binding cassette sub-family C member 8 Organism : Homo sapiens Q09428 ENSG00000006071 |
||||
|
Protein: Sulfonylurea receptor 1, Kir6.2 Description: ATP-sensitive inward rectifier potassium channel 11 Organism : Homo sapiens Q14654 ENSG00000187486 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 5441 |
| ChEMBL | CHEMBL472 |
| DrugBank | DB01016 |
| DrugCentral | 1314 |
| FDA SRS | SX6K58TVWC |
| Human Metabolome Database | HMDB0015151 |
| Guide to Pharmacology | 2414 |
| KEGG | C07022 |
| PDB | GBM |
| PharmGKB | PA449782 |
| PubChem | 3488 |
| SureChEMBL | SCHEMBL22009 |
| ZINC | ZINC000000537805 |
 Canis lupus familiaris
Canis lupus familiaris
 Cavia porcellus
Cavia porcellus
 Cricetulus griseus
Cricetulus griseus
 Homo sapiens
Homo sapiens
 Mesocricetus auratus
Mesocricetus auratus
 Mus musculus
Mus musculus
 Oryctolagus cuniculus
Oryctolagus cuniculus
 Rattus norvegicus
Rattus norvegicus