| Trade Names | |
| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| ATC | N03AX10 |
| UNII | X72RBB02N8 |
| EPA CompTox | DTXSID9023041 |
Structure
| InChI Key | WKGXYQFOCVYPAC-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C11H14N2O4 |
| Molecular Weight | 238.24 |
| AlogP | 0.96 |
| Hydrogen Bond Acceptor | 4.0 |
| Hydrogen Bond Donor | 2.0 |
| Number of Rotational Bond | 5.0 |
| Polar Surface Area | 104.64 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 1.0 |
| Heavy Atoms | 17.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Glutamate [NMDA] receptor antagonist | ANTAGONIST | FDA ISBN PubMed |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Ion channel
Ligand-gated ion channel
Ionotropic glutamate receptor
NMDA receptor
|
- | - | - | - | 61-90 |
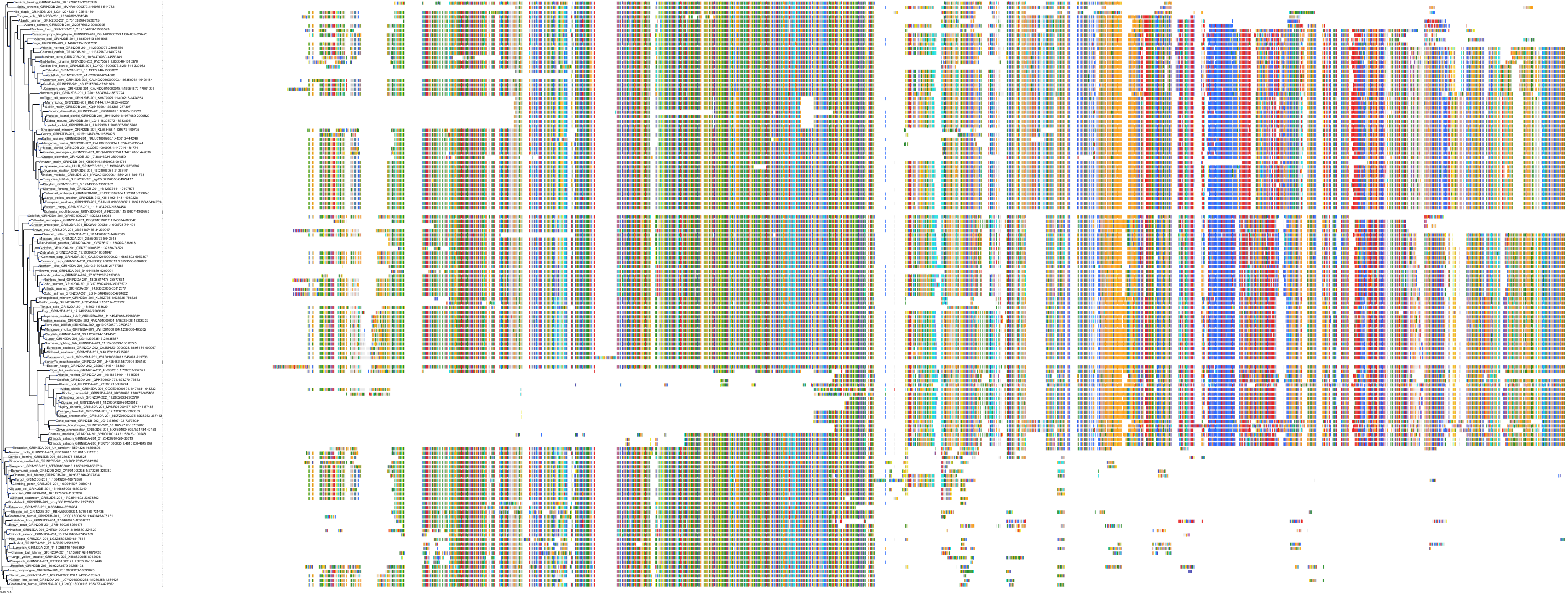
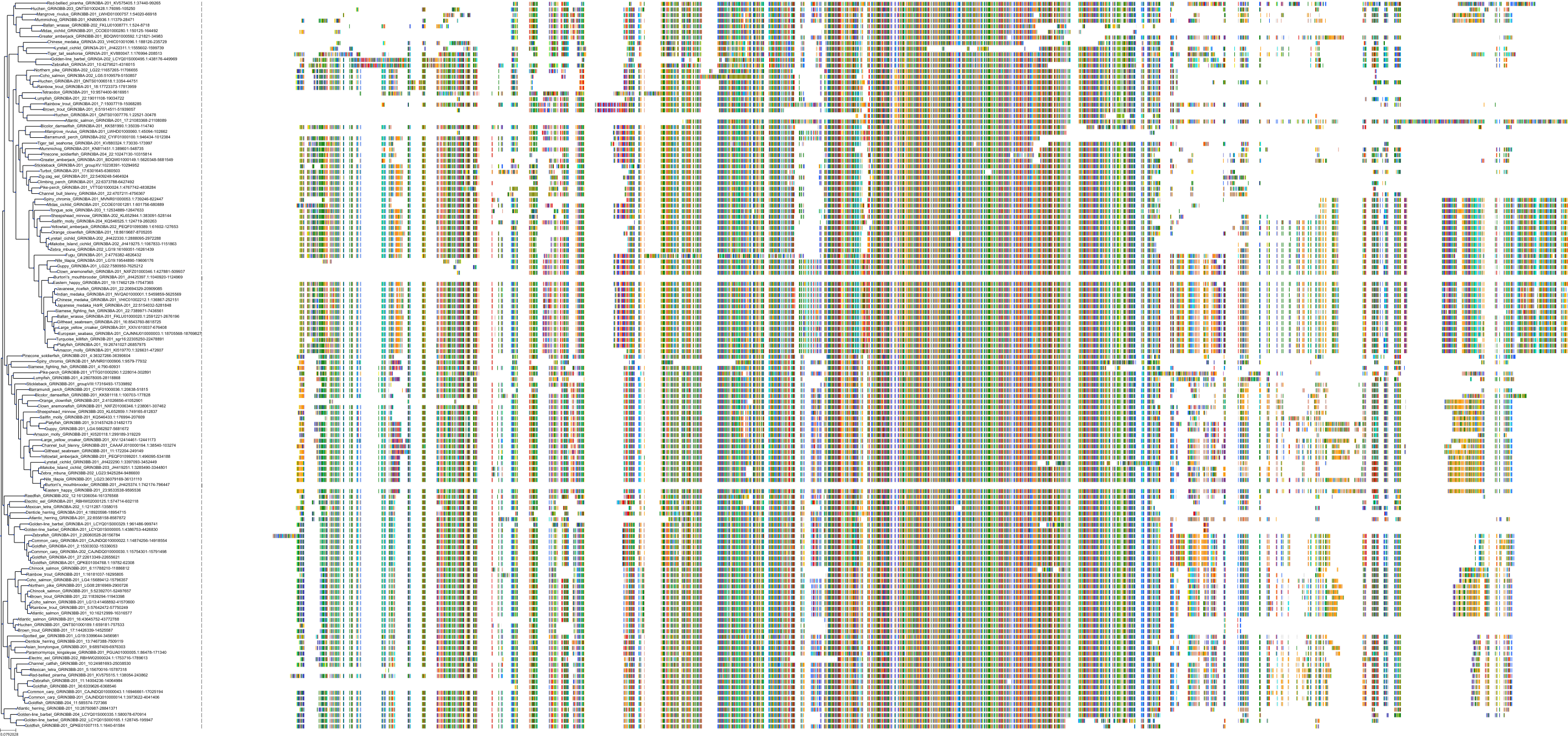
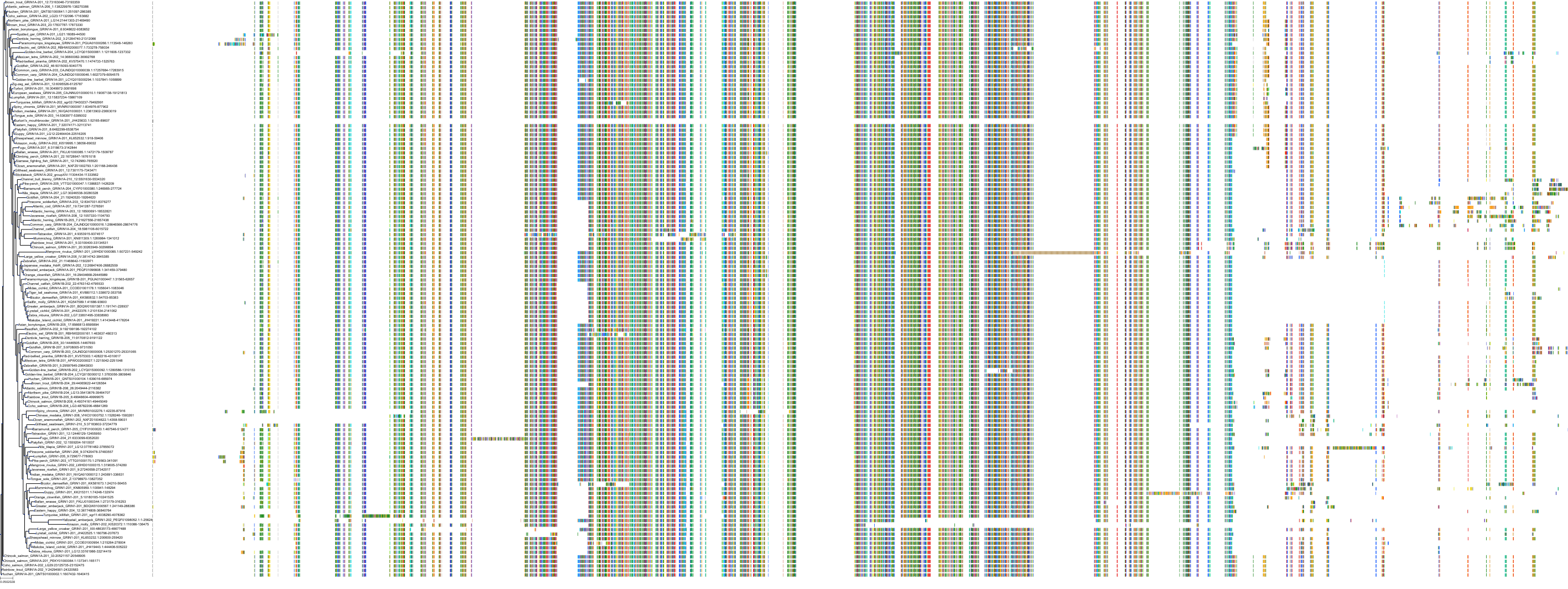
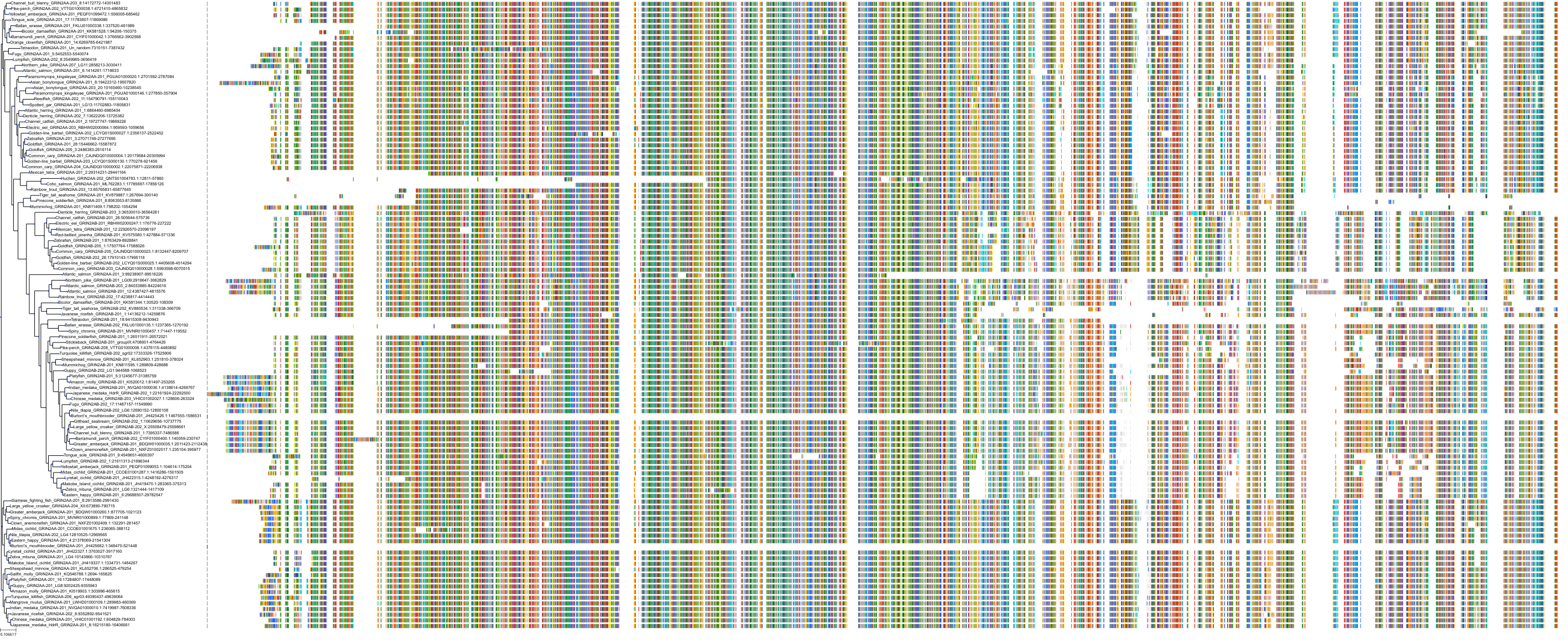
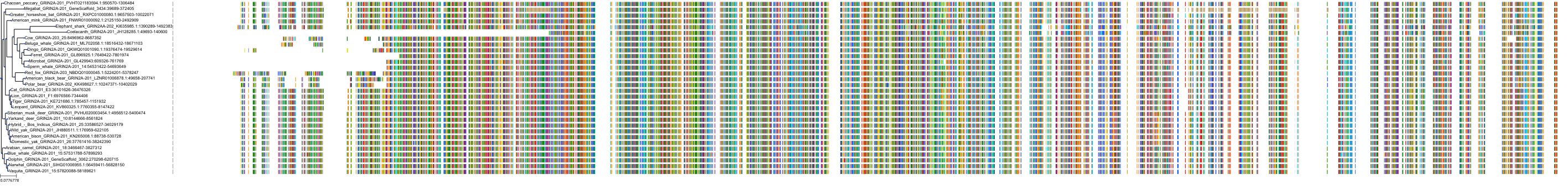
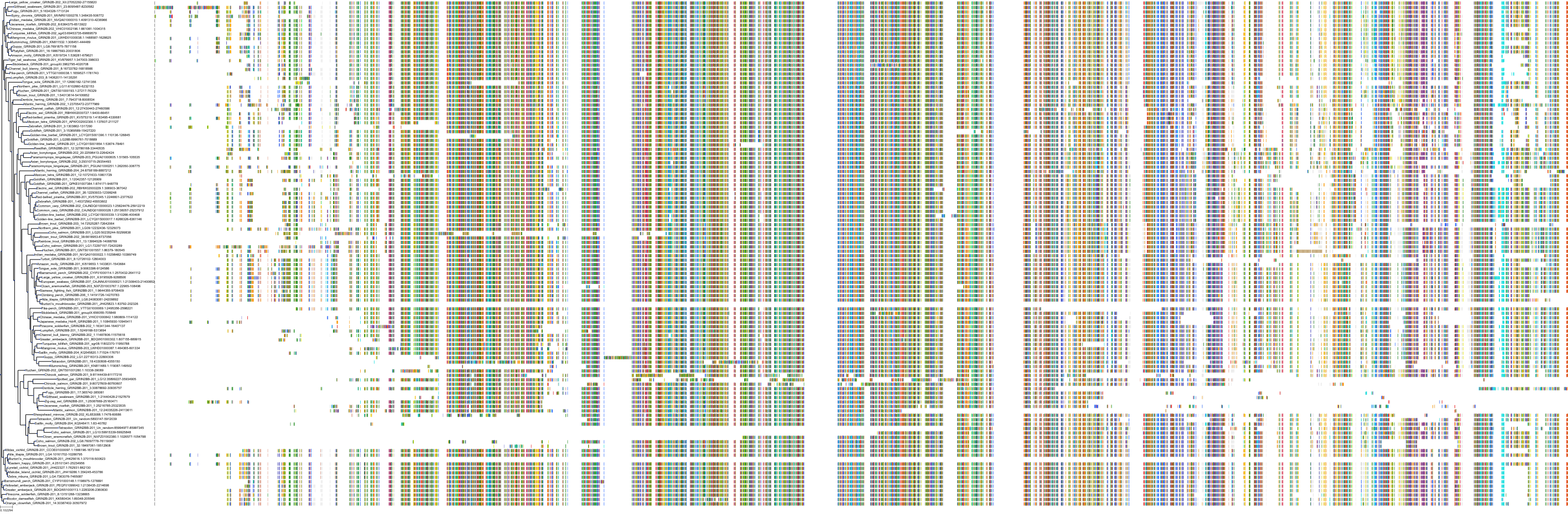
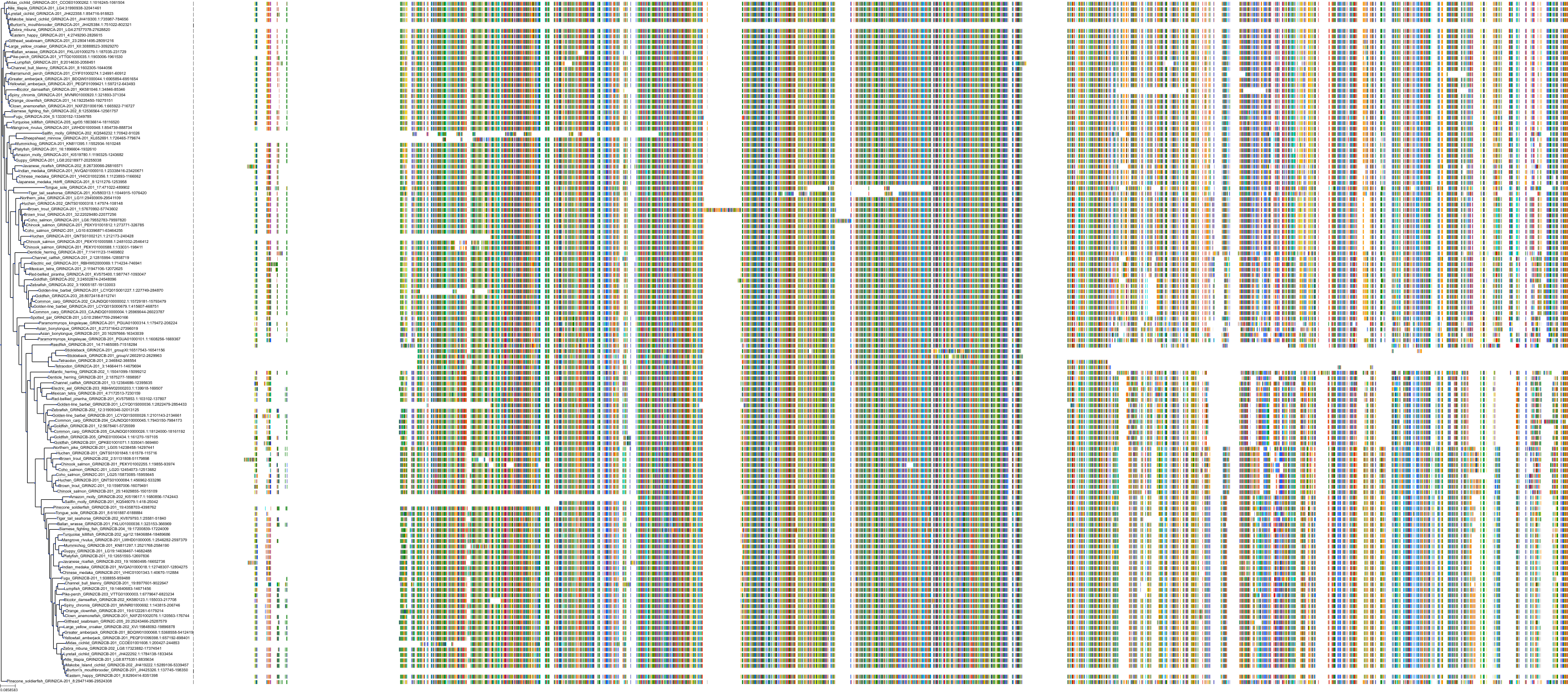
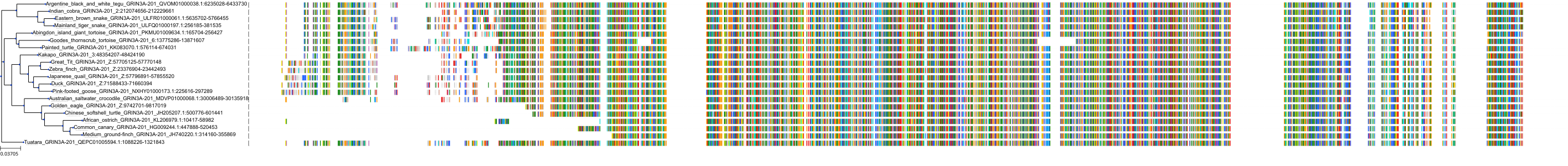
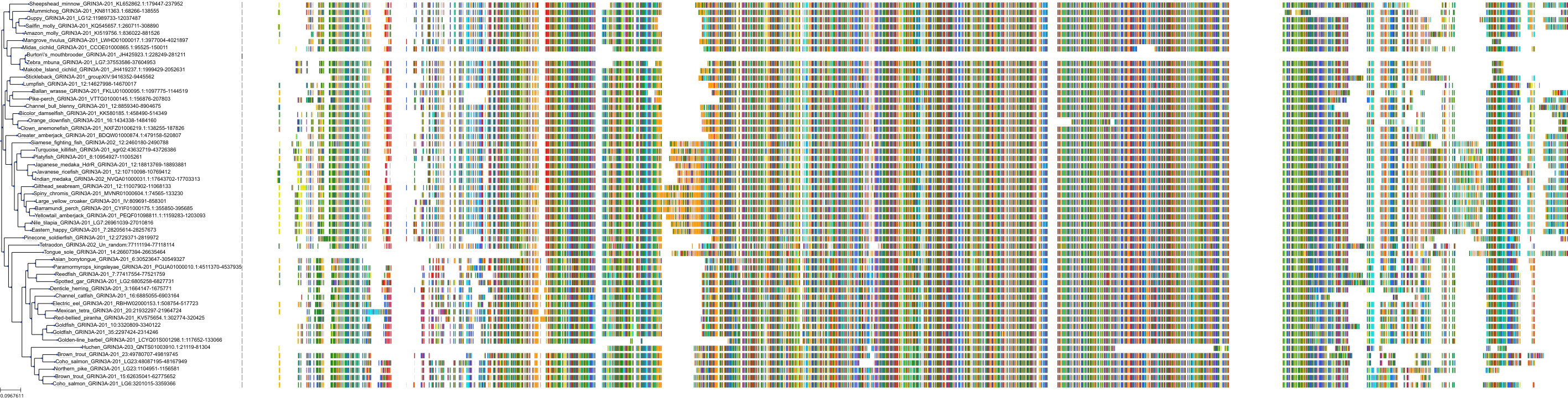
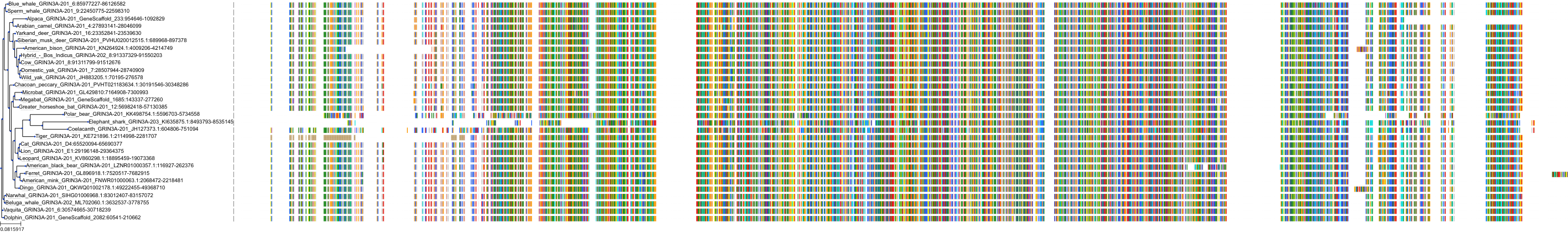
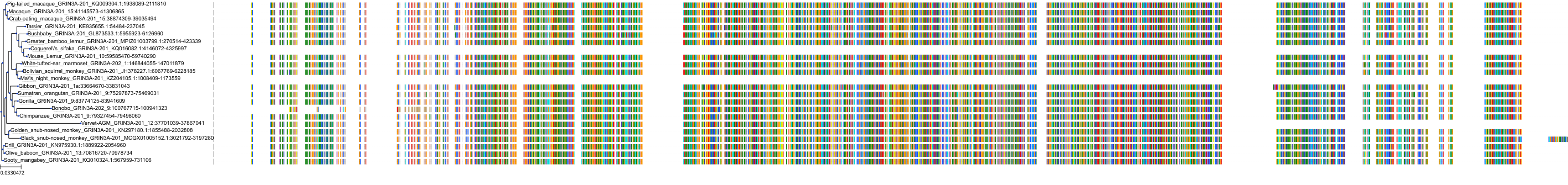
Target Conservation
|
Protein: Glutamate [NMDA] receptor Description: Glutamate receptor ionotropic, NMDA 2D Organism : Homo sapiens O15399 ENSG00000105464 |
||||
|
Protein: Glutamate [NMDA] receptor Description: Glutamate receptor ionotropic, NMDA 3B Organism : Homo sapiens O60391 ENSG00000116032 |
||||
|
Protein: Glutamate [NMDA] receptor Description: Glutamate receptor ionotropic, NMDA 1 Organism : Homo sapiens Q05586 ENSG00000176884 |
||||
|
Protein: Glutamate [NMDA] receptor Description: Glutamate receptor ionotropic, NMDA 2A Organism : Homo sapiens Q12879 ENSG00000183454 |
||||
|
Protein: Glutamate [NMDA] receptor Description: Glutamate receptor ionotropic, NMDA 2B Organism : Homo sapiens Q13224 ENSG00000273079 |
||||
|
Protein: Glutamate [NMDA] receptor Description: Glutamate receptor ionotropic, NMDA 2C Organism : Homo sapiens Q14957 ENSG00000161509 |
||||
|
Protein: Glutamate [NMDA] receptor Description: Glutamate receptor ionotropic, NMDA 3A Organism : Homo sapiens Q8TCU5 ENSG00000198785 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 4995 |
| ChEMBL | CHEMBL1094 |
| DrugBank | DB00949 |
| DrugCentral | 1140 |
| FDA SRS | X72RBB02N8 |
| Human Metabolome Database | HMDB0015084 |
| Guide to Pharmacology | 5473 |
| KEGG | C07501 |
| PharmGKB | PA449590 |
| PubChem | 3331 |
| SureChEMBL | SCHEMBL34947 |
| ZINC | ZINC000001530803 |
 Homo sapiens
Homo sapiens
 Rattus norvegicus
Rattus norvegicus