| Trade Names | |
| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| ATC | C10AX09 |
| UNII | EOR26LQQ24 |
| EPA CompTox | DTXSID1044223 |
Structure
| InChI Key | OLNTVTPDXPETLC-XPWALMASSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C24H21F2NO3 |
| Molecular Weight | 409.43 |
| AlogP | 4.89 |
| Hydrogen Bond Acceptor | 3.0 |
| Hydrogen Bond Donor | 2.0 |
| Number of Rotational Bond | 6.0 |
| Polar Surface Area | 60.77 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 3.0 |
| Heavy Atoms | 30.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Niemann-Pick C1-like protein 1 inhibitor | INHIBITOR | DailyMed |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Enzyme
Hydrolase
|
- | - | - | - | 23.5-40 | |
|
Other membrane protein
|
- | - | - | 200 | 45-80.3 | |
|
Transporter
Electrochemical transporter
SLC superfamily of solute carriers
SLC21/SLCO family of organic anion transporting polypeptides
|
- | - | - | - | -8.9-55.5 |
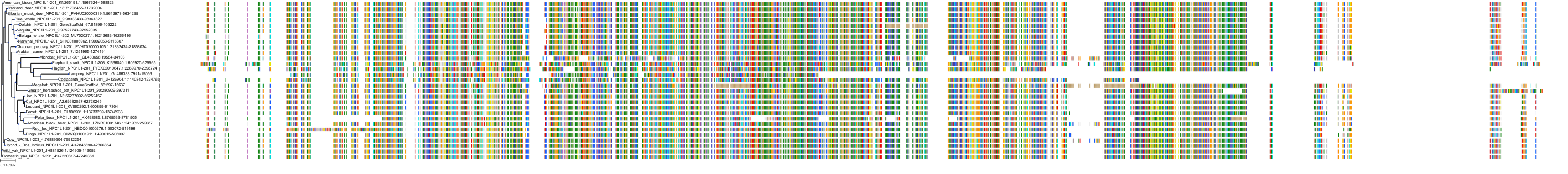
Target Conservation
|
Protein: Niemann-Pick C1-like protein 1 Description: NPC1-like intracellular cholesterol transporter 1 Organism : Homo sapiens Q9UHC9 ENSG00000015520 |
||||
Related Entries
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 49040 |
| ChEMBL | CHEMBL1138 |
| DrugBank | DB00973 |
| DrugCentral | 1125 |
| FDA SRS | EOR26LQQ24 |
| Human Metabolome Database | HMDB0015108 |
| Guide to Pharmacology | 6816 |
| KEGG | D00434 |
| PDB | H56 |
| PharmGKB | PA10816 |
| PubChem | 150311 |
| SureChEMBL | SCHEMBL2871 |
| ZINC | ZINC000003810860 |
 Cricetinae
Cricetinae
 Cricetulus griseus
Cricetulus griseus
 Homo sapiens
Homo sapiens
 Mus musculus
Mus musculus
 Oryctolagus cuniculus
Oryctolagus cuniculus
 Rattus norvegicus
Rattus norvegicus