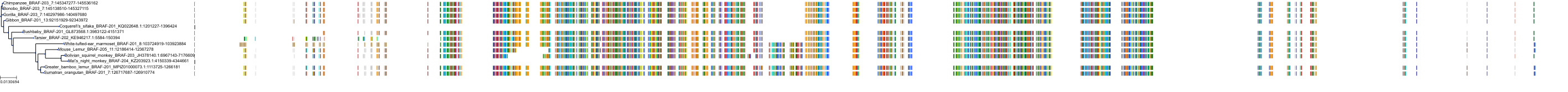| Trade Names | |
| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| ATC | L01EC03 |
| UNII | 8L7891MRB6 |
| EPA CompTox | DTXSID00155347 |
Structure
| InChI Key | CMJCXYNUCSMDBY-ZDUSSCGKSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C22H27ClFN7O4S |
| Molecular Weight | 540.02 |
| AlogP | 3.91 |
| Hydrogen Bond Acceptor | 9.0 |
| Hydrogen Bond Donor | 3.0 |
| Number of Rotational Bond | 9.0 |
| Polar Surface Area | 140.13 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 3.0 |
| Heavy Atoms | 36.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Serine/threonine-protein kinase B-raf inhibitor | INHIBITOR | DOI PubMed |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Enzyme
Kinase
Protein Kinase
TKL protein kinase group
TKL protein kinase RAF family
|
- | 0.3-4 | - | - | - |
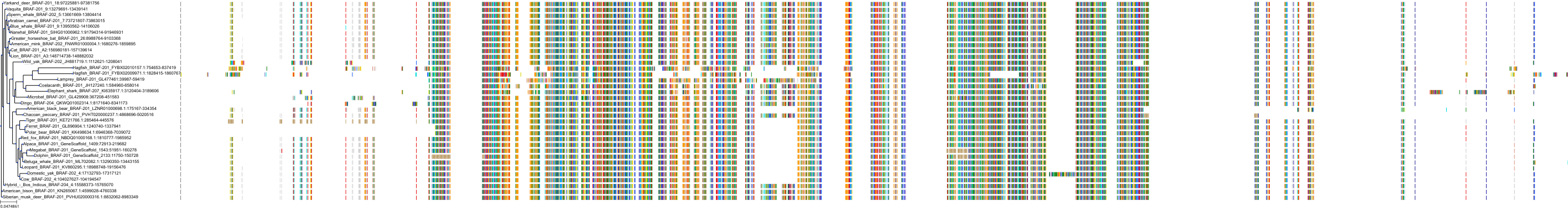
Target Conservation
|
Protein: Serine/threonine-protein kinase B-raf Description: Serine/threonine-protein kinase B-raf Organism : Homo sapiens P15056 ENSG00000157764 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL3301612 |
| DrugBank | DB11718 |
| DrugCentral | 5289 |
| FDA SRS | 8L7891MRB6 |
| Guide to Pharmacology | 7908 |
| PharmGKB | PA166179872 |
| PubChem | 50922675 |
| SureChEMBL | SCHEMBL8228295 |
| ZINC | ZINC000068249103 |
 Homo sapiens
Homo sapiens