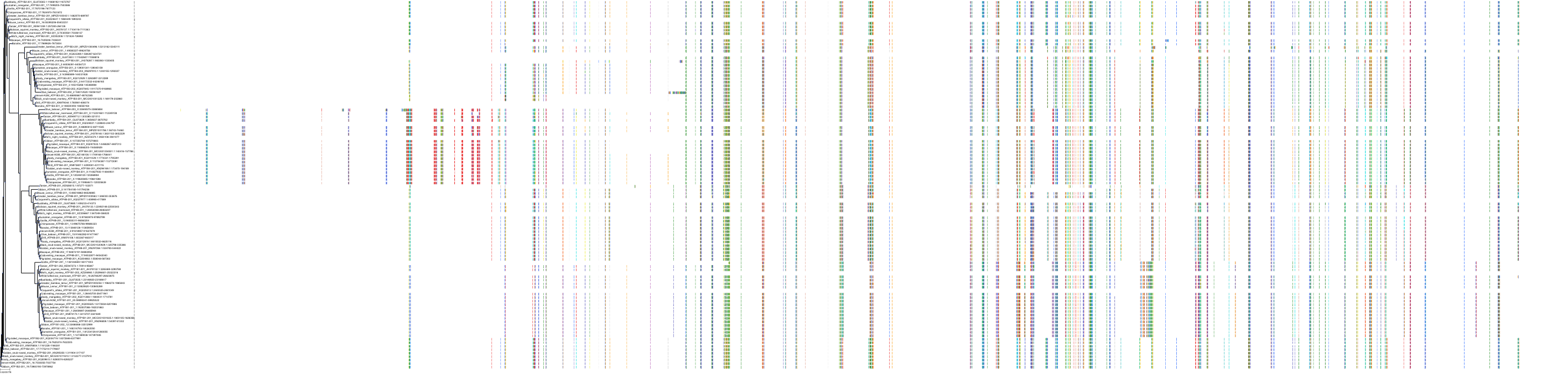
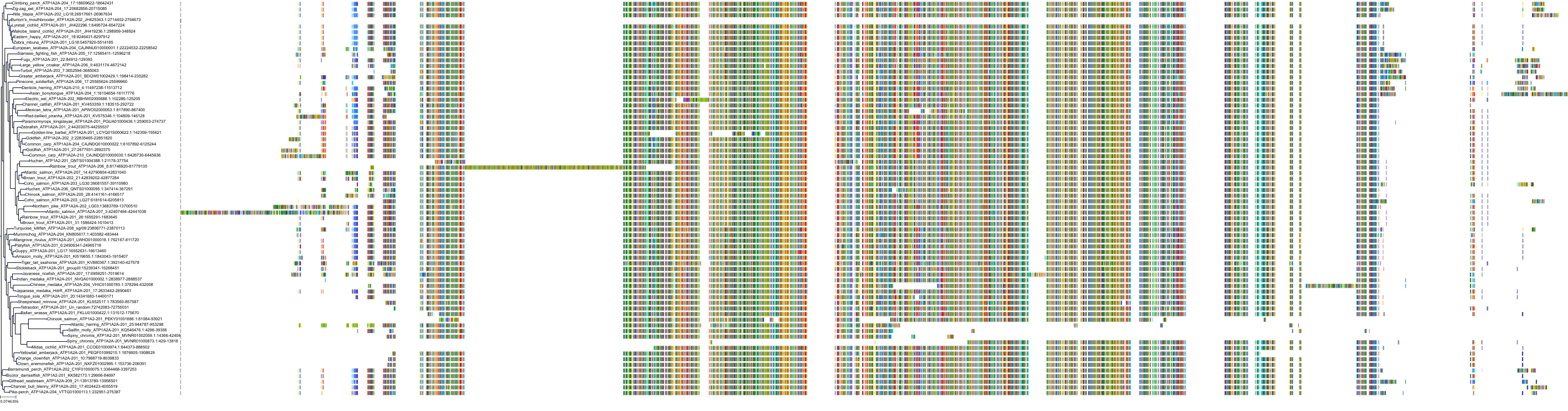
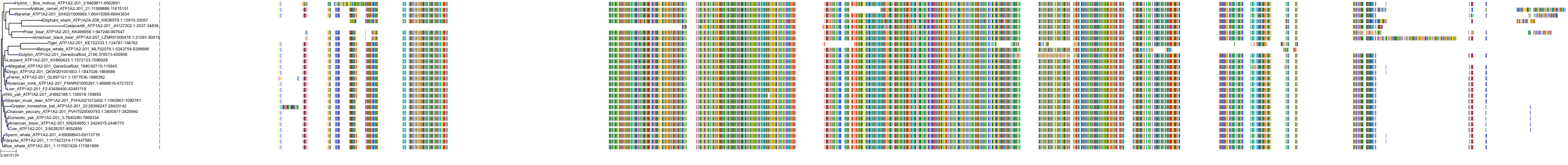
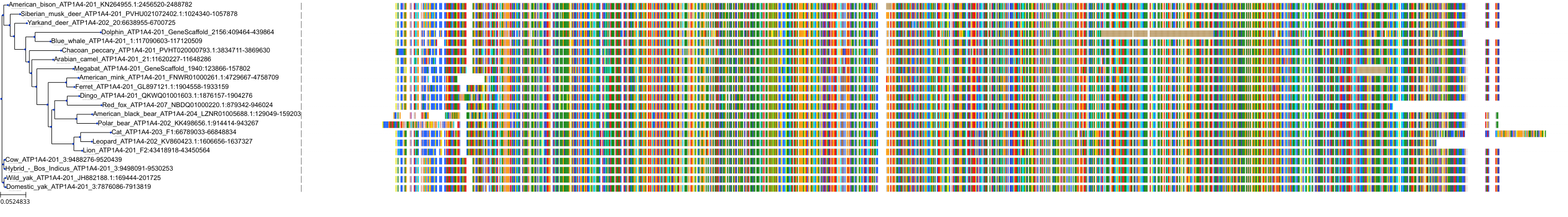
|
Inhibition of [3H]- ouabain binding to digitalis receptor
|
Cavia porcellus
|
8.0
nM
|
|
Journal : J. Med. Chem.
Title : Synthesis of 20-hydroxy-, 20-amino-, and 20-nitro-14-hydroxy-21-nor-5 beta,14 beta-pregnane C-3 glycosides and related derivatives: structure-activity relationships of pregnanes that bind to the digitalis receptor.
Year : 1993
Volume : 36
Issue : 1
First Page : 42
Last Page : 45
Authors : Templeton JF, Ling Y, Zeglam TH, LaBella FS.
Abstract : The preparation of derivatives of 14-hydroxy-21-nor-5 beta,14 beta-pregnane and 5 beta,14 beta-pregnane C-3 alpha-L-rhamnosides and tris-beta-D-digitoxosides is described. These derivatives, possessing a C-17 beta COCH2OH, CH2OH, CO2H, CO2Me, CH2NH2, or CH2NO2 group, bind to the digitalis receptor recognition site of heart muscle as measured in a radioligand binding assay. The 21-norpregnane derivatives consistently show greater binding affinity than the corresponding 20 alpha- and 20 beta-pregnane analogs. The C-20 nitro rhamnoside is comparable to digitoxin in binding affinity. The 17 beta-CH2NO2 group is the most effective replacement for the unsaturated lactone in the binding assay found so far, showing binding affinity comparable to that of the cardiac glycosides.
|
Inhibition of [3H]ouabain binding to Digitalis receptor in dog heart microsomes
|
Canis lupus familiaris
|
8.0
nM
|
|
Journal : J. Med. Chem.
Title : Synthesis and structure-activity relationships of 17beta-substituted 14beta-hydroxysteroid 3-(alpha-L-rhamnopyranoside)s: steroids that bind to the digitalis receptor.
Year : 1997
Volume : 40
Issue : 10
First Page : 1439
Last Page : 1446
Authors : Templeton JF, Ling Y, Marat K, LaBella FS.
Abstract : The preparation of 17beta-substituted 14beta-hydroxysteroid C-3 alpha-L-rhamnopyranosides is described. These derivatives have a 14beta,20-ether, 14beta,20-lactone, or 17beta-CH2CH2OH, -CH2CH2NH2, -CH=CHNO2(E), -CH=CHCOOH(E), -CH(OH)CH2NO2(R), -CH(OMe)CH2NO2(R), -CH2-CH2COOH, or -CH(OH)CH2NH2(R) group. Derivatives were assayed in a radioligand binding assay for [3H]ouabain in membranes from canine heart muscle. The digitalis "receptor" comprises isoenzymes of the ion-pumping enzyme, Na+,K+-ATPase. The 17beta-CH=CHNO2(E), 17beta-CH=CHCOOH(E), and 17beta-CH(OMe)CH2NO2(R) derivatives were the most potent and equivalent to ouabain with low-nanomolar IC50 values. The very potent binding affinity of the disubstituted compound 17beta-CH(OMe)CH2NO2(R) further demonstrates that 17beta-unsaturated substitution is not required for potent binding affinity. This observation may be of value in the separation of cardiotonic and cardiotoxic effects. Tosylation of the 17beta-CH2OH, prepared from the 17beta-CHO by lithium aluminum hydride reduction, yielded the 14beta,17beta-ether. Synthesis of the 17beta-CH2COOH gave the epimeric 14alpha,17alpha- and 14beta,17beta-lactones. Structures have been established by NMR analysis.
|
Kinetic parameter against Monoclonal antibody mAB-1B3
|
None
|
0.6
nM
|
|
Journal : J. Med. Chem.
Title : Three-dimensional quantitative structure-activity relationship analysis of ligand binding to human sequence antidigoxin monoclonal antibodies using comparative molecular field analysis.
Year : 2002
Volume : 45
Issue : 15
First Page : 3257
Last Page : 3270
Authors : Farr CD, Tabet MR, Ball WJ, Fishwild DM, Wang X, Nair AC, Welsh WJ.
Abstract : The present study indicates that the newly generated human sequence antidigoxin monoclonal antibody (mAb), 1B3, binds digoxin with a different fine specificity binding than our previously obtained human sequence monoclonal antibodies (mAbs) (Ball, W. J.; et al. J. Immunol. 1999, 163, 2291-2298). Uniquely, 1B3 has a higher affinity for digitoxin than digoxin, the immunizing hapten, and a strong requirement for at least one sugar residue linked to the aglycone (-genin). By means of comparative molecular field analysis (CoMFA), the results of competition binding studies for 56 cardiotonic and hormonal steroids were employed to develop three-dimensional quantitative structure-activity relationship (3D-QSAR) models for ligand binding to 1B3 and to three additional human sequence mAbs, as well as the murine antidigoxin mAb 40-50 (Mudgett-Hunter, M.; et al. Mol. Immunol. 1985, 22, 447-488). All five 3D-QSAR models yielded cross-validated q(2) values greater than 0.5, which indicates that they have significant predictive ability. The CoMFA StDevCoeff contour plots, as well as the competition results, indicate that 1B3 binds ligands in a manner distinct from the other four mAbs. The CoMFA contour plots for 40-50 were also compared with the known X-ray crystallographic structure of the 40-50-ouabain complex (Jeffrey, P. D.; et al. J. Mol. Biol. 1995, 248, 344-360) in order to identify correlations between residues in the mAb binding site and specific contour plot regions. These 3D-QSAR models and their respective contour plots should be useful tools to further understand the molecular nature of antibody-antigen interactions and to aid in the redesign or enhancement of therapeutic antibodies.
|
Binding affinity against Monoclonal antibody mAB-1B3 using [3H]digitoxin as radioligand
|
None
|
0.2
nM
|
|
Journal : J. Med. Chem.
Title : Three-dimensional quantitative structure-activity relationship analysis of ligand binding to human sequence antidigoxin monoclonal antibodies using comparative molecular field analysis.
Year : 2002
Volume : 45
Issue : 15
First Page : 3257
Last Page : 3270
Authors : Farr CD, Tabet MR, Ball WJ, Fishwild DM, Wang X, Nair AC, Welsh WJ.
Abstract : The present study indicates that the newly generated human sequence antidigoxin monoclonal antibody (mAb), 1B3, binds digoxin with a different fine specificity binding than our previously obtained human sequence monoclonal antibodies (mAbs) (Ball, W. J.; et al. J. Immunol. 1999, 163, 2291-2298). Uniquely, 1B3 has a higher affinity for digitoxin than digoxin, the immunizing hapten, and a strong requirement for at least one sugar residue linked to the aglycone (-genin). By means of comparative molecular field analysis (CoMFA), the results of competition binding studies for 56 cardiotonic and hormonal steroids were employed to develop three-dimensional quantitative structure-activity relationship (3D-QSAR) models for ligand binding to 1B3 and to three additional human sequence mAbs, as well as the murine antidigoxin mAb 40-50 (Mudgett-Hunter, M.; et al. Mol. Immunol. 1985, 22, 447-488). All five 3D-QSAR models yielded cross-validated q(2) values greater than 0.5, which indicates that they have significant predictive ability. The CoMFA StDevCoeff contour plots, as well as the competition results, indicate that 1B3 binds ligands in a manner distinct from the other four mAbs. The CoMFA contour plots for 40-50 were also compared with the known X-ray crystallographic structure of the 40-50-ouabain complex (Jeffrey, P. D.; et al. J. Mol. Biol. 1995, 248, 344-360) in order to identify correlations between residues in the mAb binding site and specific contour plot regions. These 3D-QSAR models and their respective contour plots should be useful tools to further understand the molecular nature of antibody-antigen interactions and to aid in the redesign or enhancement of therapeutic antibodies.
|
Binding affinity against Monoclonal antibody mAB-1B3 using [3H]digoxin as radioligand
|
None
|
0.1
nM
|
|
Journal : J. Med. Chem.
Title : Three-dimensional quantitative structure-activity relationship analysis of ligand binding to human sequence antidigoxin monoclonal antibodies using comparative molecular field analysis.
Year : 2002
Volume : 45
Issue : 15
First Page : 3257
Last Page : 3270
Authors : Farr CD, Tabet MR, Ball WJ, Fishwild DM, Wang X, Nair AC, Welsh WJ.
Abstract : The present study indicates that the newly generated human sequence antidigoxin monoclonal antibody (mAb), 1B3, binds digoxin with a different fine specificity binding than our previously obtained human sequence monoclonal antibodies (mAbs) (Ball, W. J.; et al. J. Immunol. 1999, 163, 2291-2298). Uniquely, 1B3 has a higher affinity for digitoxin than digoxin, the immunizing hapten, and a strong requirement for at least one sugar residue linked to the aglycone (-genin). By means of comparative molecular field analysis (CoMFA), the results of competition binding studies for 56 cardiotonic and hormonal steroids were employed to develop three-dimensional quantitative structure-activity relationship (3D-QSAR) models for ligand binding to 1B3 and to three additional human sequence mAbs, as well as the murine antidigoxin mAb 40-50 (Mudgett-Hunter, M.; et al. Mol. Immunol. 1985, 22, 447-488). All five 3D-QSAR models yielded cross-validated q(2) values greater than 0.5, which indicates that they have significant predictive ability. The CoMFA StDevCoeff contour plots, as well as the competition results, indicate that 1B3 binds ligands in a manner distinct from the other four mAbs. The CoMFA contour plots for 40-50 were also compared with the known X-ray crystallographic structure of the 40-50-ouabain complex (Jeffrey, P. D.; et al. J. Mol. Biol. 1995, 248, 344-360) in order to identify correlations between residues in the mAb binding site and specific contour plot regions. These 3D-QSAR models and their respective contour plots should be useful tools to further understand the molecular nature of antibody-antigen interactions and to aid in the redesign or enhancement of therapeutic antibodies.
|
Cytotoxicity against human TK10 cells after 48 hrs by SRB assay
|
Homo sapiens
|
3.2
nM
|
|
Journal : J. Nat. Prod.
Title : Digitoxin inhibits the growth of cancer cell lines at concentrations commonly found in cardiac patients.
Year : 2005
Volume : 68
Issue : 11
First Page : 1642
Last Page : 1645
Authors : López-Lázaro M, Pastor N, Azrak SS, Ayuso MJ, Austin CA, Cortés F.
Abstract : The cardiac glycosides digitoxin (1) and digoxin (3) have been used in cardiac diseases for many years. During this time several reports have suggested the possible use of digitalis in medical oncology. Several analogues of digitoxin (1) were evaluated for growth inhibition activity in three human cancer cell lines; this study showed that digitoxin (1) was the most active compound and revealed some structural features that may play a role in the growth inhibition activity of these drugs. The IC50 values for 1 (3-33 nM) were within or below the concentration range seen in the plasma of patients with cardiac disease receiving this glycoside (20-33 nM). A renal adenocarcinoma cancer cell line (TK-10) was hypersensitive to this drug, and digitoxin toxicity on these cells was mediated by apoptosis. In vitro experiments showed that 1 at 30 nM induced levels of DNA-topoisomerase II cleavable complexes similar to etoposide, a topoisomerase II poison widely used in cancer chemotherapy. Using the individual cell assay TARDIS, cells exposed to 1 for 30 min showed low but statistically significant levels of DNA-topoisomerase II cleavable complexes; however these complexes disappeared after 24 h exposure.
|
Cytotoxicity against human MCF7 cells after 48 hrs by SRB assay
|
Homo sapiens
|
10.2
nM
|
|
Journal : J. Nat. Prod.
Title : Digitoxin inhibits the growth of cancer cell lines at concentrations commonly found in cardiac patients.
Year : 2005
Volume : 68
Issue : 11
First Page : 1642
Last Page : 1645
Authors : López-Lázaro M, Pastor N, Azrak SS, Ayuso MJ, Austin CA, Cortés F.
Abstract : The cardiac glycosides digitoxin (1) and digoxin (3) have been used in cardiac diseases for many years. During this time several reports have suggested the possible use of digitalis in medical oncology. Several analogues of digitoxin (1) were evaluated for growth inhibition activity in three human cancer cell lines; this study showed that digitoxin (1) was the most active compound and revealed some structural features that may play a role in the growth inhibition activity of these drugs. The IC50 values for 1 (3-33 nM) were within or below the concentration range seen in the plasma of patients with cardiac disease receiving this glycoside (20-33 nM). A renal adenocarcinoma cancer cell line (TK-10) was hypersensitive to this drug, and digitoxin toxicity on these cells was mediated by apoptosis. In vitro experiments showed that 1 at 30 nM induced levels of DNA-topoisomerase II cleavable complexes similar to etoposide, a topoisomerase II poison widely used in cancer chemotherapy. Using the individual cell assay TARDIS, cells exposed to 1 for 30 min showed low but statistically significant levels of DNA-topoisomerase II cleavable complexes; however these complexes disappeared after 24 h exposure.
|
Cytotoxicity against human UACC62 cells after 48 hrs by SRB assay
|
Homo sapiens
|
33.5
nM
|
|
Journal : J. Nat. Prod.
Title : Digitoxin inhibits the growth of cancer cell lines at concentrations commonly found in cardiac patients.
Year : 2005
Volume : 68
Issue : 11
First Page : 1642
Last Page : 1645
Authors : López-Lázaro M, Pastor N, Azrak SS, Ayuso MJ, Austin CA, Cortés F.
Abstract : The cardiac glycosides digitoxin (1) and digoxin (3) have been used in cardiac diseases for many years. During this time several reports have suggested the possible use of digitalis in medical oncology. Several analogues of digitoxin (1) were evaluated for growth inhibition activity in three human cancer cell lines; this study showed that digitoxin (1) was the most active compound and revealed some structural features that may play a role in the growth inhibition activity of these drugs. The IC50 values for 1 (3-33 nM) were within or below the concentration range seen in the plasma of patients with cardiac disease receiving this glycoside (20-33 nM). A renal adenocarcinoma cancer cell line (TK-10) was hypersensitive to this drug, and digitoxin toxicity on these cells was mediated by apoptosis. In vitro experiments showed that 1 at 30 nM induced levels of DNA-topoisomerase II cleavable complexes similar to etoposide, a topoisomerase II poison widely used in cancer chemotherapy. Using the individual cell assay TARDIS, cells exposed to 1 for 30 min showed low but statistically significant levels of DNA-topoisomerase II cleavable complexes; however these complexes disappeared after 24 h exposure.
|
Cytotoxicity against human K562 cells by XTT assay
|
Homo sapiens
|
6.4
nM
|
|
Journal : J. Nat. Prod.
Title : Digitoxin inhibits the growth of cancer cell lines at concentrations commonly found in cardiac patients.
Year : 2005
Volume : 68
Issue : 11
First Page : 1642
Last Page : 1645
Authors : López-Lázaro M, Pastor N, Azrak SS, Ayuso MJ, Austin CA, Cortés F.
Abstract : The cardiac glycosides digitoxin (1) and digoxin (3) have been used in cardiac diseases for many years. During this time several reports have suggested the possible use of digitalis in medical oncology. Several analogues of digitoxin (1) were evaluated for growth inhibition activity in three human cancer cell lines; this study showed that digitoxin (1) was the most active compound and revealed some structural features that may play a role in the growth inhibition activity of these drugs. The IC50 values for 1 (3-33 nM) were within or below the concentration range seen in the plasma of patients with cardiac disease receiving this glycoside (20-33 nM). A renal adenocarcinoma cancer cell line (TK-10) was hypersensitive to this drug, and digitoxin toxicity on these cells was mediated by apoptosis. In vitro experiments showed that 1 at 30 nM induced levels of DNA-topoisomerase II cleavable complexes similar to etoposide, a topoisomerase II poison widely used in cancer chemotherapy. Using the individual cell assay TARDIS, cells exposed to 1 for 30 min showed low but statistically significant levels of DNA-topoisomerase II cleavable complexes; however these complexes disappeared after 24 h exposure.
|
Cytotoxicity against human DU145 cells after 72 hrs by calcein AM assay
|
Homo sapiens
|
440.0
nM
|
|
Journal : J. Nat. Prod.
Title : Neoglycorandomization and chemoenzymatic glycorandomization: two complementary tools for natural product diversification.
Year : 2005
Volume : 68
Issue : 11
First Page : 1696
Last Page : 1711
Authors : Langenhan JM, Griffith BR, Thorson JS.
Abstract : In an effort to explore the contribution of the sugar constituents of pharmaceutically relevant glycosylated natural products, chemists have developed glycosylation methods that are amenable to the generation of libraries of analogues with a broad array of glycosidic attachments. Recently, two complementary glycorandomization strategies have been described, namely, neoglycorandomization, a chemical approach based on a one-step sugar ligation reaction that does not require any prior sugar protection or activation, and chemoenzymatic glycorandomization, a biocatalytic approach that relies on the substrate promiscuity of enzymes to activate and attach sugars to natural products. Since both methods require reducing sugars, this review first highlights recent advances in monosaccharide generation and then follows with an overview of recent progress in the development of neoglycorandomization and chemoenzymatic glycorandomization.
|
Cytotoxicity against human MCF7 cells after 72 hrs by calcein AM assay
|
Homo sapiens
|
440.0
nM
|
|
Journal : J. Nat. Prod.
Title : Neoglycorandomization and chemoenzymatic glycorandomization: two complementary tools for natural product diversification.
Year : 2005
Volume : 68
Issue : 11
First Page : 1696
Last Page : 1711
Authors : Langenhan JM, Griffith BR, Thorson JS.
Abstract : In an effort to explore the contribution of the sugar constituents of pharmaceutically relevant glycosylated natural products, chemists have developed glycosylation methods that are amenable to the generation of libraries of analogues with a broad array of glycosidic attachments. Recently, two complementary glycorandomization strategies have been described, namely, neoglycorandomization, a chemical approach based on a one-step sugar ligation reaction that does not require any prior sugar protection or activation, and chemoenzymatic glycorandomization, a biocatalytic approach that relies on the substrate promiscuity of enzymes to activate and attach sugars to natural products. Since both methods require reducing sugars, this review first highlights recent advances in monosaccharide generation and then follows with an overview of recent progress in the development of neoglycorandomization and chemoenzymatic glycorandomization.
|
Cytotoxicity against human HCT116 cells after 72 hrs by calcein AM assay
|
Homo sapiens
|
440.0
nM
|
|
Journal : J. Nat. Prod.
Title : Neoglycorandomization and chemoenzymatic glycorandomization: two complementary tools for natural product diversification.
Year : 2005
Volume : 68
Issue : 11
First Page : 1696
Last Page : 1711
Authors : Langenhan JM, Griffith BR, Thorson JS.
Abstract : In an effort to explore the contribution of the sugar constituents of pharmaceutically relevant glycosylated natural products, chemists have developed glycosylation methods that are amenable to the generation of libraries of analogues with a broad array of glycosidic attachments. Recently, two complementary glycorandomization strategies have been described, namely, neoglycorandomization, a chemical approach based on a one-step sugar ligation reaction that does not require any prior sugar protection or activation, and chemoenzymatic glycorandomization, a biocatalytic approach that relies on the substrate promiscuity of enzymes to activate and attach sugars to natural products. Since both methods require reducing sugars, this review first highlights recent advances in monosaccharide generation and then follows with an overview of recent progress in the development of neoglycorandomization and chemoenzymatic glycorandomization.
|
Cytotoxicity against human Hep3B cells after 72 hrs by calcein AM assay
|
Homo sapiens
|
440.0
nM
|
|
Journal : J. Nat. Prod.
Title : Neoglycorandomization and chemoenzymatic glycorandomization: two complementary tools for natural product diversification.
Year : 2005
Volume : 68
Issue : 11
First Page : 1696
Last Page : 1711
Authors : Langenhan JM, Griffith BR, Thorson JS.
Abstract : In an effort to explore the contribution of the sugar constituents of pharmaceutically relevant glycosylated natural products, chemists have developed glycosylation methods that are amenable to the generation of libraries of analogues with a broad array of glycosidic attachments. Recently, two complementary glycorandomization strategies have been described, namely, neoglycorandomization, a chemical approach based on a one-step sugar ligation reaction that does not require any prior sugar protection or activation, and chemoenzymatic glycorandomization, a biocatalytic approach that relies on the substrate promiscuity of enzymes to activate and attach sugars to natural products. Since both methods require reducing sugars, this review first highlights recent advances in monosaccharide generation and then follows with an overview of recent progress in the development of neoglycorandomization and chemoenzymatic glycorandomization.
|
Cytotoxicity against human SF268 cells after 72 hrs by calcein AM assay
|
Homo sapiens
|
440.0
nM
|
|
Journal : J. Nat. Prod.
Title : Neoglycorandomization and chemoenzymatic glycorandomization: two complementary tools for natural product diversification.
Year : 2005
Volume : 68
Issue : 11
First Page : 1696
Last Page : 1711
Authors : Langenhan JM, Griffith BR, Thorson JS.
Abstract : In an effort to explore the contribution of the sugar constituents of pharmaceutically relevant glycosylated natural products, chemists have developed glycosylation methods that are amenable to the generation of libraries of analogues with a broad array of glycosidic attachments. Recently, two complementary glycorandomization strategies have been described, namely, neoglycorandomization, a chemical approach based on a one-step sugar ligation reaction that does not require any prior sugar protection or activation, and chemoenzymatic glycorandomization, a biocatalytic approach that relies on the substrate promiscuity of enzymes to activate and attach sugars to natural products. Since both methods require reducing sugars, this review first highlights recent advances in monosaccharide generation and then follows with an overview of recent progress in the development of neoglycorandomization and chemoenzymatic glycorandomization.
|
Cytotoxicity against human SKOV3 cells after 72 hrs by calcein AM assay
|
Homo sapiens
|
440.0
nM
|
|
Journal : J. Nat. Prod.
Title : Neoglycorandomization and chemoenzymatic glycorandomization: two complementary tools for natural product diversification.
Year : 2005
Volume : 68
Issue : 11
First Page : 1696
Last Page : 1711
Authors : Langenhan JM, Griffith BR, Thorson JS.
Abstract : In an effort to explore the contribution of the sugar constituents of pharmaceutically relevant glycosylated natural products, chemists have developed glycosylation methods that are amenable to the generation of libraries of analogues with a broad array of glycosidic attachments. Recently, two complementary glycorandomization strategies have been described, namely, neoglycorandomization, a chemical approach based on a one-step sugar ligation reaction that does not require any prior sugar protection or activation, and chemoenzymatic glycorandomization, a biocatalytic approach that relies on the substrate promiscuity of enzymes to activate and attach sugars to natural products. Since both methods require reducing sugars, this review first highlights recent advances in monosaccharide generation and then follows with an overview of recent progress in the development of neoglycorandomization and chemoenzymatic glycorandomization.
|
Cytotoxicity against human NCI-H460 cells after 72 hrs by calcein AM assay
|
Homo sapiens
|
440.0
nM
|
|
Journal : J. Nat. Prod.
Title : Neoglycorandomization and chemoenzymatic glycorandomization: two complementary tools for natural product diversification.
Year : 2005
Volume : 68
Issue : 11
First Page : 1696
Last Page : 1711
Authors : Langenhan JM, Griffith BR, Thorson JS.
Abstract : In an effort to explore the contribution of the sugar constituents of pharmaceutically relevant glycosylated natural products, chemists have developed glycosylation methods that are amenable to the generation of libraries of analogues with a broad array of glycosidic attachments. Recently, two complementary glycorandomization strategies have been described, namely, neoglycorandomization, a chemical approach based on a one-step sugar ligation reaction that does not require any prior sugar protection or activation, and chemoenzymatic glycorandomization, a biocatalytic approach that relies on the substrate promiscuity of enzymes to activate and attach sugars to natural products. Since both methods require reducing sugars, this review first highlights recent advances in monosaccharide generation and then follows with an overview of recent progress in the development of neoglycorandomization and chemoenzymatic glycorandomization.
|
Cytotoxicity against human A549 cells after 72 hrs by calcein AM assay
|
Homo sapiens
|
440.0
nM
|
|
Journal : J. Nat. Prod.
Title : Neoglycorandomization and chemoenzymatic glycorandomization: two complementary tools for natural product diversification.
Year : 2005
Volume : 68
Issue : 11
First Page : 1696
Last Page : 1711
Authors : Langenhan JM, Griffith BR, Thorson JS.
Abstract : In an effort to explore the contribution of the sugar constituents of pharmaceutically relevant glycosylated natural products, chemists have developed glycosylation methods that are amenable to the generation of libraries of analogues with a broad array of glycosidic attachments. Recently, two complementary glycorandomization strategies have been described, namely, neoglycorandomization, a chemical approach based on a one-step sugar ligation reaction that does not require any prior sugar protection or activation, and chemoenzymatic glycorandomization, a biocatalytic approach that relies on the substrate promiscuity of enzymes to activate and attach sugars to natural products. Since both methods require reducing sugars, this review first highlights recent advances in monosaccharide generation and then follows with an overview of recent progress in the development of neoglycorandomization and chemoenzymatic glycorandomization.
|
Cytotoxicity against human NCI/ADR-RES cells after 72 hrs by calcein AM assay
|
Homo sapiens
|
440.0
nM
|
|
Journal : J. Nat. Prod.
Title : Neoglycorandomization and chemoenzymatic glycorandomization: two complementary tools for natural product diversification.
Year : 2005
Volume : 68
Issue : 11
First Page : 1696
Last Page : 1711
Authors : Langenhan JM, Griffith BR, Thorson JS.
Abstract : In an effort to explore the contribution of the sugar constituents of pharmaceutically relevant glycosylated natural products, chemists have developed glycosylation methods that are amenable to the generation of libraries of analogues with a broad array of glycosidic attachments. Recently, two complementary glycorandomization strategies have been described, namely, neoglycorandomization, a chemical approach based on a one-step sugar ligation reaction that does not require any prior sugar protection or activation, and chemoenzymatic glycorandomization, a biocatalytic approach that relies on the substrate promiscuity of enzymes to activate and attach sugars to natural products. Since both methods require reducing sugars, this review first highlights recent advances in monosaccharide generation and then follows with an overview of recent progress in the development of neoglycorandomization and chemoenzymatic glycorandomization.
|
Cytotoxicity against human CC20 cells after 72 hrs by FMCA method
|
Homo sapiens
|
410.0
nM
|
|
Journal : J. Nat. Prod.
Title : Cytotoxic effects of cardiac glycosides in colon cancer cells, alone and in combination with standard chemotherapeutic drugs.
Year : 2009
Volume : 72
Issue : 11
First Page : 1969
Last Page : 1974
Authors : Felth J, Rickardson L, Rosén J, Wickström M, Fryknäs M, Lindskog M, Bohlin L, Gullbo J.
Abstract : Cardiac glycosides have been reported to exhibit cytotoxic activity against several different cancer types, but studies against colorectal cancer are lacking. In a screening procedure aimed at identifying natural products with activity against colon cancer, several cardiac glycosides were shown to be of interest, and five of these were further evaluated in different colorectal cancer cell lines and primary cells from patients. Convallatoxin (1), oleandrin (4), and proscillaridin A (5) were identified as the most potent compounds (submicromolar IC50 values), and digitoxin (2) and digoxin (3), which are used in cardiac disease, exhibited somewhat lower activity (IC50 values 0.27-4.1 microM). Selected cardiac glycosides were tested in combination with four clinically relevant cytotoxic drugs (5-fluorouracil, oxaliplatin, cisplatin, irinotecan). The combination of 2 and oxaliplatin exhibited synergism including the otherwise highly drug-resistant HT29 cell line. A ChemGPS-NP application comparing modes of action of anticancer drugs identified cardiac glycosides as a separate cluster. These findings demonstrate that such substances may exhibit significant activity against colorectal cancer cell lines, by mechanisms disparate from currently used anticancer drugs, but at concentrations generally considered not achievable in patient plasma.
|
Cytotoxicity against human HCT116 cells after 72 hrs by FMCA method
|
Homo sapiens
|
740.0
nM
|
|
Journal : J. Nat. Prod.
Title : Cytotoxic effects of cardiac glycosides in colon cancer cells, alone and in combination with standard chemotherapeutic drugs.
Year : 2009
Volume : 72
Issue : 11
First Page : 1969
Last Page : 1974
Authors : Felth J, Rickardson L, Rosén J, Wickström M, Fryknäs M, Lindskog M, Bohlin L, Gullbo J.
Abstract : Cardiac glycosides have been reported to exhibit cytotoxic activity against several different cancer types, but studies against colorectal cancer are lacking. In a screening procedure aimed at identifying natural products with activity against colon cancer, several cardiac glycosides were shown to be of interest, and five of these were further evaluated in different colorectal cancer cell lines and primary cells from patients. Convallatoxin (1), oleandrin (4), and proscillaridin A (5) were identified as the most potent compounds (submicromolar IC50 values), and digitoxin (2) and digoxin (3), which are used in cardiac disease, exhibited somewhat lower activity (IC50 values 0.27-4.1 microM). Selected cardiac glycosides were tested in combination with four clinically relevant cytotoxic drugs (5-fluorouracil, oxaliplatin, cisplatin, irinotecan). The combination of 2 and oxaliplatin exhibited synergism including the otherwise highly drug-resistant HT29 cell line. A ChemGPS-NP application comparing modes of action of anticancer drugs identified cardiac glycosides as a separate cluster. These findings demonstrate that such substances may exhibit significant activity against colorectal cancer cell lines, by mechanisms disparate from currently used anticancer drugs, but at concentrations generally considered not achievable in patient plasma.
|
Cytotoxicity against human colon cancer cells after 72 hrs by FMCA method
|
Homo sapiens
|
100.0
nM
|
|
Journal : J. Nat. Prod.
Title : Cytotoxic effects of cardiac glycosides in colon cancer cells, alone and in combination with standard chemotherapeutic drugs.
Year : 2009
Volume : 72
Issue : 11
First Page : 1969
Last Page : 1974
Authors : Felth J, Rickardson L, Rosén J, Wickström M, Fryknäs M, Lindskog M, Bohlin L, Gullbo J.
Abstract : Cardiac glycosides have been reported to exhibit cytotoxic activity against several different cancer types, but studies against colorectal cancer are lacking. In a screening procedure aimed at identifying natural products with activity against colon cancer, several cardiac glycosides were shown to be of interest, and five of these were further evaluated in different colorectal cancer cell lines and primary cells from patients. Convallatoxin (1), oleandrin (4), and proscillaridin A (5) were identified as the most potent compounds (submicromolar IC50 values), and digitoxin (2) and digoxin (3), which are used in cardiac disease, exhibited somewhat lower activity (IC50 values 0.27-4.1 microM). Selected cardiac glycosides were tested in combination with four clinically relevant cytotoxic drugs (5-fluorouracil, oxaliplatin, cisplatin, irinotecan). The combination of 2 and oxaliplatin exhibited synergism including the otherwise highly drug-resistant HT29 cell line. A ChemGPS-NP application comparing modes of action of anticancer drugs identified cardiac glycosides as a separate cluster. These findings demonstrate that such substances may exhibit significant activity against colorectal cancer cell lines, by mechanisms disparate from currently used anticancer drugs, but at concentrations generally considered not achievable in patient plasma.
|
PUBCHEM_BIOASSAY: Dose response cell-based assay to measure STAT3 inhibition. (Class of assay: confirmatory) [Related pubchem assays: 920, 1317, 1265, 1308, 862 ]
|
Homo sapiens
|
700.0
nM
|
|
Title : PubChem BioAssay data set
|
Cytotoxicity against human NCI-H460 cells assessed as cell death at 50 nM after 12 hrs by Hoechst-33342 staining and propidium iodide staining
|
Homo sapiens
|
357.0
nM
|
|
Journal : ACS Med. Chem. Lett.
Title : Stereochemical survey of digitoxin monosaccharides: new anticancer analogues with enhanced apoptotic activity and growth inhibitory effect on human non-small cell lung cancer cell.
Year : 2011
Volume : 2
Issue : 1
First Page : 73
Last Page : 78
Authors : Wang HY, Xin W, Zhou M, Stueckle TA, Rojanasakul Y, O'Doherty GA.
Abstract : A stereochemically diverse array of monosaccharide analogues of the trisaccharide based cardiac glycoside natural product digitoxin has been synthesized using a de novo asymmetric approach. The analogues were tested for cytotoxicity against the NCI panel of 60 human cancer cell lines and in more detail against non-small cell human lung cancer cells (NCI-H460). The results were compared with digitoxin and its aglycone digitoxigenin. Three novel digitoxin monosaccharide analogues with β-d-digitoxose, α-l-rhamnose, and α-l-amicetose sugar moieties showed excellent selectivity and activity. Further investigation revealed that digitoxin α-l-rhamnose and α-l-amicetose analogues displayed similar anti-proliferation effects, but with at least 5-fold greater potency in apoptosis induction than digitoxin against NCI-H460. This study demonstrates the ability to improve the digitoxin anti-cancer activity by modification of the stereochemistry and substitution of the carbohydrate moiety of this known cardiac drug.
|
Antiproliferative activity against human MCF7 cells assessed as cell viability treated for 48 hrs followed by compound washout measured after 72 hrs by fluorometric CellTiter-Blue reagent assay
|
Homo sapiens
|
20.0
nM
|
|
Journal : Bioorg. Med. Chem.
Title : Synthesis and evaluation of cardiac glycoside mimics as potential anticancer drugs.
Year : 2011
Volume : 19
Issue : 7
First Page : 2407
Last Page : 2417
Authors : Jensen M, Schmidt S, Fedosova NU, Mollenhauer J, Jensen HH.
Abstract : The cardiac glycoside digitoxin, consisting of a steroid core linked to a labile trisaccharide, has been used for centuries for the treatment of congestive heart failure. The well known pharmacological effect is a result of the ability of cardiac glycosides to inhibit the Na(+), K(+)-ATPase. Within recent years cardiac glycosides have furthermore been suggested to possess valuable anticancer activity. To mimic the labile trisaccharide of digitoxin with a stabile carbohydrate surrogate, we have used sulfur linked ethylene glycol moieties of varying length (mono-, di-, tri- or tetra-ethylene glycol), and furthermore used these linkers as handles for the synthesis of bivalent steroids. The prepared compounds were evaluated for their potencies to inhibit the Na(+), K(+)-ATPase and for their cytotoxic effect on cancerous MCF-7 cells. A clear trend is observed in both inhibition and cytotoxic effect, where the bioactivity decreases as the size increases. The most potent Na(+), K(+)-ATPase inhibitors are the compounds with the shortest ethylene glycol chain (K(app) 0.48 μM) and thiodigitoxigenin (K(app) 0.42 μM), which both are comparable with digitoxigenin (K(app) 0.52 μM). For the cancer cell viability assay the shortest mimics were found to have highest efficacy, with the best ligand having a monoethylene glycol unit (IC(50) 0.24 μM), which was slightly better than digitoxigenin (IC(50) 0.64 μM), while none of the novel cardiac glycoside mimics display an in vitro effect as high as digitoxin (IC(50) 0.02 μM).
|
DRUGMATRIX: ATPase, Na+/K+ enzyme inhibition (substrate: ATP)
|
Sus scrofa
|
225.0
nM
|
|
Title : DrugMatrix in vitro pharmacology data
Authors : Scott S. Auerbach, DrugMatrix¨ and ToxFX¨ Coordinator National Toxicology Program
Abstract : The DrugMatrix Pharmacology data is a subset of the data freely available from the National Toxicology Program. For more details see:https://ntp.niehs.nih.gov/drugmatrix/index.html
|
TP_TRANSPORTER: inhibition of Digoxin uptake in OATP4C1-expressing MDCK cells
|
None
|
120.0
nM
|
|
Journal : Proc. Natl. Acad. Sci. U.S.A.
Title : Isolation and characterization of a digoxin transporter and its rat homologue expressed in the kidney.
Year : 2004
Volume : 101
Issue : 1
First Page : 3569
Last Page : 3574
Authors : Mikkaichi T, Suzuki T, Onogawa T, Tanemoto M, Mizutamari H, Okada M, Chaki T, Masuda S, Tokui T, Eto N, Abe M, Satoh F, Unno M, Hishinuma T, Inui K, Ito S, Goto J, Abe T.
Abstract : Digoxin, which is one of the most commonly prescribed drugs for the treatment of heart failure, is mainly eliminated from the circulation by the kidney. P-glycoprotein is well characterized as a digoxin pump at the apical membrane of the nephron. However, little is known about the transport mechanism at the basolateral membrane. We have isolated an organic anion transporter (OATP4C1) from human kidney. Human OATP4C1 is the first member of the organic anion transporting polypeptide (OATP) family expressed in human kidney. The isolated cDNA encodes a polypeptide of 724 aa with 12 transmembrane domains. The genomic organization consists of 13 exons located on chromosome 5q21. Its rat counterpart, Oatp4c1, is also isolated from rat kidney. Human OATP4C1 transports cardiac glycosides (digoxin, K(m) = 7.8 microM and ouabain, K(m) = 0.38 microM), thyroid hormone (triiodothyronine, K(m) = 5.9 microM and thyroxine), cAMP, and methotrexate in a sodium-independent manner. Rat Oatp4c1 also transports digoxin (K(m) = 8.0 microM) and triiodothyronine (K(m) = 1.9 microM). Immunohistochemical analysis reveals that rat Oatp4c1 protein is localized at the basolateral membrane of the proximal tubule cell in the kidney. These data suggest that human OATP4C1/rat Oatp4c1 might be a first step of the transport pathway of digoxin and various compounds into urine in the kidney.
|
Inhibition of electric eel AChE at 2 mg/ml by Ellman's method
|
Electrophorus electricus
|
-1.83
%
|
|
Journal : Bioorg. Med. Chem.
Title : Exploration of natural compounds as sources of new bifunctional scaffolds targeting cholinesterases and beta amyloid aggregation: the case of chelerythrine.
Year : 2012
Volume : 20
Issue : 22
First Page : 6669
Last Page : 6679
Authors : Brunhofer G, Fallarero A, Karlsson D, Batista-Gonzalez A, Shinde P, Gopi Mohan C, Vuorela P.
Abstract : The presented project started by screening a library consisting of natural and natural based compounds for their acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitory activity. Active compounds were chemically clustered into groups and further tested on the human cholinesterases isoforms. The aim of the presented study was to identify compounds that could be used as leads to target two key mechanisms associated with the AD's pathogenesis simultaneously: cholinergic depletion and beta amyloid (Aβ) aggregation. Berberin, palmatine and chelerythrine, chemically clustered in the so-called isoquinoline group, showed promising cholinesterase inhibitory activity and were therefore further investigated. Moreover, the compounds demonstrated moderate to good inhibition of Aβ aggregation as well as the ability to disaggregate already preformed Aβ aggregates in an experimental set-up using HFIP as promotor of Aβ aggregates. Analysis of the kinetic mechanism of the AChE inhibition revealed chelerythrine as a mixed inhibitor. Using molecular docking studies, it was further proven that chelerythrine binds on both the catalytic site and the peripheral anionic site (PAS) of the AChE. In view of this, we went on to investigate its effect on inhibiting Aβ aggregation stimulated by AChE. Chelerythrine showed inhibition of fibril formation in the same range as propidium iodide. This approach enabled for the first time to identify a cholinesterase inhibitor of natural origin-chelerythrine-acting on AChE and BChE with a dual ability to inhibit Aβ aggregation as well as to disaggregate preformed Aβ aggregates. This compound could be an excellent starting point paving the way to develop more successful anti-AD drugs.
|
Inhibition of horse BChE at 2 mg/ml by Ellman's method
|
Equus caballus
|
-0.78
%
|
|
Journal : Bioorg. Med. Chem.
Title : Exploration of natural compounds as sources of new bifunctional scaffolds targeting cholinesterases and beta amyloid aggregation: the case of chelerythrine.
Year : 2012
Volume : 20
Issue : 22
First Page : 6669
Last Page : 6679
Authors : Brunhofer G, Fallarero A, Karlsson D, Batista-Gonzalez A, Shinde P, Gopi Mohan C, Vuorela P.
Abstract : The presented project started by screening a library consisting of natural and natural based compounds for their acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitory activity. Active compounds were chemically clustered into groups and further tested on the human cholinesterases isoforms. The aim of the presented study was to identify compounds that could be used as leads to target two key mechanisms associated with the AD's pathogenesis simultaneously: cholinergic depletion and beta amyloid (Aβ) aggregation. Berberin, palmatine and chelerythrine, chemically clustered in the so-called isoquinoline group, showed promising cholinesterase inhibitory activity and were therefore further investigated. Moreover, the compounds demonstrated moderate to good inhibition of Aβ aggregation as well as the ability to disaggregate already preformed Aβ aggregates in an experimental set-up using HFIP as promotor of Aβ aggregates. Analysis of the kinetic mechanism of the AChE inhibition revealed chelerythrine as a mixed inhibitor. Using molecular docking studies, it was further proven that chelerythrine binds on both the catalytic site and the peripheral anionic site (PAS) of the AChE. In view of this, we went on to investigate its effect on inhibiting Aβ aggregation stimulated by AChE. Chelerythrine showed inhibition of fibril formation in the same range as propidium iodide. This approach enabled for the first time to identify a cholinesterase inhibitor of natural origin-chelerythrine-acting on AChE and BChE with a dual ability to inhibit Aβ aggregation as well as to disaggregate preformed Aβ aggregates. This compound could be an excellent starting point paving the way to develop more successful anti-AD drugs.
|
Inhibition of sodium fluorescein uptake in OATP1B1-transfected CHO cells at an equimolar substrate-inhibitor concentration of 10 uM
|
Cricetulus griseus
|
56.48
%
|
|
Journal : Mol. Pharmacol.
Title : Structure-based identification of OATP1B1/3 inhibitors.
Year : 2013
Volume : 83
Issue : 6
First Page : 1257
Last Page : 1267
Authors : De Bruyn T, van Westen GJ, Ijzerman AP, Stieger B, de Witte P, Augustijns PF, Annaert PP.
Abstract : Several recent studies show that inhibition of the hepatic transport proteins organic anion-transporting polypeptide 1B1 (OATP1B1) and 1B3 (OATP1B3) can result in clinically relevant drug-drug interactions (DDI). To avoid late-stage development drug failures due to OATP1B-mediated DDI, predictive in vitro and in silico methods should be implemented at an early stage of the drug candidate evaluation process. In the present study, we first developed a high-throughput in vitro transporter inhibition assay for the OATP1B subfamily. A total of 2000 compounds were tested as potential modulators of the uptake of the OATP1B substrate sodium fluorescein, in OATP1B1- or 1B3-transfected Chinese hamster ovary cells. At an equimolar substrate-inhibitor concentration of 10 µM, 212 and 139 molecules were identified as OATP1B1 and OATP1B3 inhibitors, respectively (minimum 50% inhibition). For 69 compounds, previously not identified as OATP1B inhibitors, concentration-dependent inhibition was also determined, yielding Ki values ranging from 0.06 to 6.5 µM. Based on these in vitro data, we subsequently developed a proteochemometrics-based in silico model, which predicted OATP1B inhibitors in the test group (20% of the dataset) with high specificity (86%) and sensitivity (78%). Moreover, several physicochemical compound properties and substructures related to OATP1B1/1B3 inhibition or inactivity were identified. Finally, model performance was prospectively verified with a set of 54 compounds not included in the original dataset. This validation indicated that 80 and 74% of the compounds were correctly classified for OATP1B1 and OATP1B3 inhibition, respectively.
|
Inhibition of sodium fluorescein uptake in OATP1B3-transfected CHO cells at an equimolar substrate-inhibitor concentration of 10 uM
|
Cricetulus griseus
|
66.85
%
|
|
Journal : Mol. Pharmacol.
Title : Structure-based identification of OATP1B1/3 inhibitors.
Year : 2013
Volume : 83
Issue : 6
First Page : 1257
Last Page : 1267
Authors : De Bruyn T, van Westen GJ, Ijzerman AP, Stieger B, de Witte P, Augustijns PF, Annaert PP.
Abstract : Several recent studies show that inhibition of the hepatic transport proteins organic anion-transporting polypeptide 1B1 (OATP1B1) and 1B3 (OATP1B3) can result in clinically relevant drug-drug interactions (DDI). To avoid late-stage development drug failures due to OATP1B-mediated DDI, predictive in vitro and in silico methods should be implemented at an early stage of the drug candidate evaluation process. In the present study, we first developed a high-throughput in vitro transporter inhibition assay for the OATP1B subfamily. A total of 2000 compounds were tested as potential modulators of the uptake of the OATP1B substrate sodium fluorescein, in OATP1B1- or 1B3-transfected Chinese hamster ovary cells. At an equimolar substrate-inhibitor concentration of 10 µM, 212 and 139 molecules were identified as OATP1B1 and OATP1B3 inhibitors, respectively (minimum 50% inhibition). For 69 compounds, previously not identified as OATP1B inhibitors, concentration-dependent inhibition was also determined, yielding Ki values ranging from 0.06 to 6.5 µM. Based on these in vitro data, we subsequently developed a proteochemometrics-based in silico model, which predicted OATP1B inhibitors in the test group (20% of the dataset) with high specificity (86%) and sensitivity (78%). Moreover, several physicochemical compound properties and substructures related to OATP1B1/1B3 inhibition or inactivity were identified. Finally, model performance was prospectively verified with a set of 54 compounds not included in the original dataset. This validation indicated that 80 and 74% of the compounds were correctly classified for OATP1B1 and OATP1B3 inhibition, respectively.
|
Antiviral activity against Human cytomegalovirus Towne infected in HFF assessed as inhibition of viral replication by measuring pp28 expression at 72 hrs post-infection by luciferase assay
|
Human herpesvirus 5 strain Towne
|
23.3
nM
|
|
Journal : ACS Med. Chem. Lett.
Title : Digitoxin analogues with improved anticytomegalovirus activity.
Year : 2014
Volume : 5
Issue : 4
First Page : 395
Last Page : 399
Authors : Cai H, Wang HY, Venkatadri R, Fu DX, Forman M, Bajaj SO, Li H, O'Doherty GA, Arav-Boger R.
Abstract : Cardiac glycosides are potent inhibitors of cancer cell growth and possess antiviral activities at nanomolar concentrations. In this study we evaluated the anticytomegalovirus (CMV) activity of digitoxin and several of its analogues. We show that sugar type and sugar length attached to the steroid core structure affects its anticytomegalovirus activity. Structure-activity relationship (SAR) studies identified the l-sugar containing cardiac glycosides as having improved anti-CMV activity and may lead to better understanding of how these compounds inhibit CMV replication.
|
Cytotoxicity against HFF assessed as inhibition of cell proliferation at 0.1 uM after 3 days by MTT assay
|
Homo sapiens
|
50.0
%
|
|
Journal : ACS Med. Chem. Lett.
Title : Digitoxin analogues with improved anticytomegalovirus activity.
Year : 2014
Volume : 5
Issue : 4
First Page : 395
Last Page : 399
Authors : Cai H, Wang HY, Venkatadri R, Fu DX, Forman M, Bajaj SO, Li H, O'Doherty GA, Arav-Boger R.
Abstract : Cardiac glycosides are potent inhibitors of cancer cell growth and possess antiviral activities at nanomolar concentrations. In this study we evaluated the anticytomegalovirus (CMV) activity of digitoxin and several of its analogues. We show that sugar type and sugar length attached to the steroid core structure affects its anticytomegalovirus activity. Structure-activity relationship (SAR) studies identified the l-sugar containing cardiac glycosides as having improved anti-CMV activity and may lead to better understanding of how these compounds inhibit CMV replication.
|
Inhibition of rat brain sodium/potassium-transporting ATPase in presence of Mg2+ and Na+
|
Rattus norvegicus
|
700.0
nM
|
|
Journal : J. Med. Chem.
Title : Cardenolide analogues. 2. 22-Methylenecard-14-enolides.
Year : 1977
Volume : 20
Issue : 6
First Page : 841
Last Page : 844
Authors : Fullerton DS, Gilman TM, Pankaskie MC, Ahmed K, From AH, Duax WL, Rohrer DC.
Abstract : 22-Methylene-3beta-hydroxy-5beta,20(S)-card-14-enolide (11) and 22-methylene-3beta-hydroxy-5beta,20(R)-card-14-enolide (12) were synthesized from digitoxin (1). Attempts to prepare the 14beta-hydroxy-22-methylene analogues were unsuccessful. The 20(R) isomer (12) was found in Na+, K+-ATPase inhibition studies to be twice as active as 14-dehydrogitoxigenin (17). The 20(S) isomer (11) was significantly less active than 17. The hydrolysis of steroid 3beta-tert-butyldimethysilyl ethers was also found to be much more difficult than with nonsteroids.
|
Inhibition of rat brain sodium/potassium-transporting ATPase after 10 mins in presence of Mg2+ and Na+
|
Rattus norvegicus
|
240.0
nM
|
|
Journal : J. Med. Chem.
Title : Cardenolide analogues. 2. 22-Methylenecard-14-enolides.
Year : 1977
Volume : 20
Issue : 6
First Page : 841
Last Page : 844
Authors : Fullerton DS, Gilman TM, Pankaskie MC, Ahmed K, From AH, Duax WL, Rohrer DC.
Abstract : 22-Methylene-3beta-hydroxy-5beta,20(S)-card-14-enolide (11) and 22-methylene-3beta-hydroxy-5beta,20(R)-card-14-enolide (12) were synthesized from digitoxin (1). Attempts to prepare the 14beta-hydroxy-22-methylene analogues were unsuccessful. The 20(R) isomer (12) was found in Na+, K+-ATPase inhibition studies to be twice as active as 14-dehydrogitoxigenin (17). The 20(S) isomer (11) was significantly less active than 17. The hydrolysis of steroid 3beta-tert-butyldimethysilyl ethers was also found to be much more difficult than with nonsteroids.
|
PubChem BioAssay. VEGF stimulated ADSC/ECFC co-culture nuclear area decrease (viability)-IC50. (Class of assay: confirmatory)
|
None
|
103.11
nM
|
|
Title : PubChem BioAssay data set
|
PubChem BioAssay. VEGF stimulated ADSC/ECFC co-culture CD31-stained tube area decrease-IC50. (Class of assay: confirmatory)
|
None
|
11.0
nM
|
|
Title : PubChem BioAssay data set
|
Antiproliferative activity against human OVCAR4 cells after 72 hrs by CellTiter 96 aqueous one solution assay
|
Homo sapiens
|
425.6
nM
|
|
Journal : J Nat Prod
Title : (+)-Strebloside-Induced Cytotoxicity in Ovarian Cancer Cells Is Mediated through Cardiac Glycoside Signaling Networks.
Year : 2017
Volume : 80
Issue : 3
First Page : 659
Last Page : 669
Authors : Chen WL, Ren Y, Ren J, Erxleben C, Johnson ME, Gentile S, Kinghorn AD, Swanson SM, Burdette JE.
Abstract : (+)-Strebloside, a cardiac glycoside isolated from the stem bark of Streblus asper collected in Vietnam, has shown some potential for further investigation as an antineoplastic agent. A mechanistic study using an in vitro assay and molecular docking analysis indicated that (+)-strebloside binds and inhibits Na+/K+-ATPase in a similar manner to digitoxin. Inhibition of growth of different high-grade serous ovarian cancer cells including OVCAR3, OVSAHO, Kuramochi, OVCAR4, OVCAR5, and OVCAR8 resulted from treatment with (+)-strebloside. Furthermore, this compound blocked cell cycle progression at the G2 phase and induced PARP cleavage, indicating apoptosis activation in OVCAR3 cells. (+)-Strebloside potently inhibited mutant p53 expression through the induction of ERK pathways and inhibited NF-κB activity in human ovarian cancer cells. However, in spite of its antitumor potential, the overall biological activity of (+)-strebloside must be regarded as being typical of better-known cardiac glycosides such as digoxin and ouabain. Further chemical alteration of cardiac glycosides might help to reduce negative side effects while increasing cancer cell cytotoxicity.
|
Antiproliferative activity against human OVCAR5 cells after 72 hrs by CellTiter 96 aqueous one solution assay
|
Homo sapiens
|
321.0
nM
|
|
Journal : J Nat Prod
Title : (+)-Strebloside-Induced Cytotoxicity in Ovarian Cancer Cells Is Mediated through Cardiac Glycoside Signaling Networks.
Year : 2017
Volume : 80
Issue : 3
First Page : 659
Last Page : 669
Authors : Chen WL, Ren Y, Ren J, Erxleben C, Johnson ME, Gentile S, Kinghorn AD, Swanson SM, Burdette JE.
Abstract : (+)-Strebloside, a cardiac glycoside isolated from the stem bark of Streblus asper collected in Vietnam, has shown some potential for further investigation as an antineoplastic agent. A mechanistic study using an in vitro assay and molecular docking analysis indicated that (+)-strebloside binds and inhibits Na+/K+-ATPase in a similar manner to digitoxin. Inhibition of growth of different high-grade serous ovarian cancer cells including OVCAR3, OVSAHO, Kuramochi, OVCAR4, OVCAR5, and OVCAR8 resulted from treatment with (+)-strebloside. Furthermore, this compound blocked cell cycle progression at the G2 phase and induced PARP cleavage, indicating apoptosis activation in OVCAR3 cells. (+)-Strebloside potently inhibited mutant p53 expression through the induction of ERK pathways and inhibited NF-κB activity in human ovarian cancer cells. However, in spite of its antitumor potential, the overall biological activity of (+)-strebloside must be regarded as being typical of better-known cardiac glycosides such as digoxin and ouabain. Further chemical alteration of cardiac glycosides might help to reduce negative side effects while increasing cancer cell cytotoxicity.
|
Antiproliferative activity against human IOSE-80 cells after 72 hrs by CellTiter 96 aqueous one solution assay
|
Homo sapiens
|
48.4
nM
|
|
Journal : J Nat Prod
Title : (+)-Strebloside-Induced Cytotoxicity in Ovarian Cancer Cells Is Mediated through Cardiac Glycoside Signaling Networks.
Year : 2017
Volume : 80
Issue : 3
First Page : 659
Last Page : 669
Authors : Chen WL, Ren Y, Ren J, Erxleben C, Johnson ME, Gentile S, Kinghorn AD, Swanson SM, Burdette JE.
Abstract : (+)-Strebloside, a cardiac glycoside isolated from the stem bark of Streblus asper collected in Vietnam, has shown some potential for further investigation as an antineoplastic agent. A mechanistic study using an in vitro assay and molecular docking analysis indicated that (+)-strebloside binds and inhibits Na+/K+-ATPase in a similar manner to digitoxin. Inhibition of growth of different high-grade serous ovarian cancer cells including OVCAR3, OVSAHO, Kuramochi, OVCAR4, OVCAR5, and OVCAR8 resulted from treatment with (+)-strebloside. Furthermore, this compound blocked cell cycle progression at the G2 phase and induced PARP cleavage, indicating apoptosis activation in OVCAR3 cells. (+)-Strebloside potently inhibited mutant p53 expression through the induction of ERK pathways and inhibited NF-κB activity in human ovarian cancer cells. However, in spite of its antitumor potential, the overall biological activity of (+)-strebloside must be regarded as being typical of better-known cardiac glycosides such as digoxin and ouabain. Further chemical alteration of cardiac glycosides might help to reduce negative side effects while increasing cancer cell cytotoxicity.
|
Antiproliferative activity against human OVCAR8 cells after 72 hrs by CellTiter 96 aqueous one solution assay
|
Homo sapiens
|
71.5
nM
|
|
Journal : J Nat Prod
Title : (+)-Strebloside-Induced Cytotoxicity in Ovarian Cancer Cells Is Mediated through Cardiac Glycoside Signaling Networks.
Year : 2017
Volume : 80
Issue : 3
First Page : 659
Last Page : 669
Authors : Chen WL, Ren Y, Ren J, Erxleben C, Johnson ME, Gentile S, Kinghorn AD, Swanson SM, Burdette JE.
Abstract : (+)-Strebloside, a cardiac glycoside isolated from the stem bark of Streblus asper collected in Vietnam, has shown some potential for further investigation as an antineoplastic agent. A mechanistic study using an in vitro assay and molecular docking analysis indicated that (+)-strebloside binds and inhibits Na+/K+-ATPase in a similar manner to digitoxin. Inhibition of growth of different high-grade serous ovarian cancer cells including OVCAR3, OVSAHO, Kuramochi, OVCAR4, OVCAR5, and OVCAR8 resulted from treatment with (+)-strebloside. Furthermore, this compound blocked cell cycle progression at the G2 phase and induced PARP cleavage, indicating apoptosis activation in OVCAR3 cells. (+)-Strebloside potently inhibited mutant p53 expression through the induction of ERK pathways and inhibited NF-κB activity in human ovarian cancer cells. However, in spite of its antitumor potential, the overall biological activity of (+)-strebloside must be regarded as being typical of better-known cardiac glycosides such as digoxin and ouabain. Further chemical alteration of cardiac glycosides might help to reduce negative side effects while increasing cancer cell cytotoxicity.
|
Antiproliferative activity against human MDA-MB-435 cells after 72 hrs by CellTiter 96 aqueous one solution assay
|
Homo sapiens
|
43.3
nM
|
|
Journal : J Nat Prod
Title : (+)-Strebloside-Induced Cytotoxicity in Ovarian Cancer Cells Is Mediated through Cardiac Glycoside Signaling Networks.
Year : 2017
Volume : 80
Issue : 3
First Page : 659
Last Page : 669
Authors : Chen WL, Ren Y, Ren J, Erxleben C, Johnson ME, Gentile S, Kinghorn AD, Swanson SM, Burdette JE.
Abstract : (+)-Strebloside, a cardiac glycoside isolated from the stem bark of Streblus asper collected in Vietnam, has shown some potential for further investigation as an antineoplastic agent. A mechanistic study using an in vitro assay and molecular docking analysis indicated that (+)-strebloside binds and inhibits Na+/K+-ATPase in a similar manner to digitoxin. Inhibition of growth of different high-grade serous ovarian cancer cells including OVCAR3, OVSAHO, Kuramochi, OVCAR4, OVCAR5, and OVCAR8 resulted from treatment with (+)-strebloside. Furthermore, this compound blocked cell cycle progression at the G2 phase and induced PARP cleavage, indicating apoptosis activation in OVCAR3 cells. (+)-Strebloside potently inhibited mutant p53 expression through the induction of ERK pathways and inhibited NF-κB activity in human ovarian cancer cells. However, in spite of its antitumor potential, the overall biological activity of (+)-strebloside must be regarded as being typical of better-known cardiac glycosides such as digoxin and ouabain. Further chemical alteration of cardiac glycosides might help to reduce negative side effects while increasing cancer cell cytotoxicity.
|
Antiproliferative activity against human MDA-MB-231 cells after 72 hrs by CellTiter 96 aqueous one solution assay
|
Homo sapiens
|
482.0
nM
|
|
Journal : J Nat Prod
Title : (+)-Strebloside-Induced Cytotoxicity in Ovarian Cancer Cells Is Mediated through Cardiac Glycoside Signaling Networks.
Year : 2017
Volume : 80
Issue : 3
First Page : 659
Last Page : 669
Authors : Chen WL, Ren Y, Ren J, Erxleben C, Johnson ME, Gentile S, Kinghorn AD, Swanson SM, Burdette JE.
Abstract : (+)-Strebloside, a cardiac glycoside isolated from the stem bark of Streblus asper collected in Vietnam, has shown some potential for further investigation as an antineoplastic agent. A mechanistic study using an in vitro assay and molecular docking analysis indicated that (+)-strebloside binds and inhibits Na+/K+-ATPase in a similar manner to digitoxin. Inhibition of growth of different high-grade serous ovarian cancer cells including OVCAR3, OVSAHO, Kuramochi, OVCAR4, OVCAR5, and OVCAR8 resulted from treatment with (+)-strebloside. Furthermore, this compound blocked cell cycle progression at the G2 phase and induced PARP cleavage, indicating apoptosis activation in OVCAR3 cells. (+)-Strebloside potently inhibited mutant p53 expression through the induction of ERK pathways and inhibited NF-κB activity in human ovarian cancer cells. However, in spite of its antitumor potential, the overall biological activity of (+)-strebloside must be regarded as being typical of better-known cardiac glycosides such as digoxin and ouabain. Further chemical alteration of cardiac glycosides might help to reduce negative side effects while increasing cancer cell cytotoxicity.
|
Antiproliferative activity against human HT-29 cells after 72 hrs by CellTiter 96 aqueous one solution assay
|
Homo sapiens
|
67.6
nM
|
|
Journal : J Nat Prod
Title : (+)-Strebloside-Induced Cytotoxicity in Ovarian Cancer Cells Is Mediated through Cardiac Glycoside Signaling Networks.
Year : 2017
Volume : 80
Issue : 3
First Page : 659
Last Page : 669
Authors : Chen WL, Ren Y, Ren J, Erxleben C, Johnson ME, Gentile S, Kinghorn AD, Swanson SM, Burdette JE.
Abstract : (+)-Strebloside, a cardiac glycoside isolated from the stem bark of Streblus asper collected in Vietnam, has shown some potential for further investigation as an antineoplastic agent. A mechanistic study using an in vitro assay and molecular docking analysis indicated that (+)-strebloside binds and inhibits Na+/K+-ATPase in a similar manner to digitoxin. Inhibition of growth of different high-grade serous ovarian cancer cells including OVCAR3, OVSAHO, Kuramochi, OVCAR4, OVCAR5, and OVCAR8 resulted from treatment with (+)-strebloside. Furthermore, this compound blocked cell cycle progression at the G2 phase and induced PARP cleavage, indicating apoptosis activation in OVCAR3 cells. (+)-Strebloside potently inhibited mutant p53 expression through the induction of ERK pathways and inhibited NF-κB activity in human ovarian cancer cells. However, in spite of its antitumor potential, the overall biological activity of (+)-strebloside must be regarded as being typical of better-known cardiac glycosides such as digoxin and ouabain. Further chemical alteration of cardiac glycosides might help to reduce negative side effects while increasing cancer cell cytotoxicity.
|
Antiproliferative activity against human OVCAR3 cells after 72 hrs by CellTiter 96 aqueous one solution assay
|
Homo sapiens
|
117.1
nM
|
|
Journal : J Nat Prod
Title : (+)-Strebloside-Induced Cytotoxicity in Ovarian Cancer Cells Is Mediated through Cardiac Glycoside Signaling Networks.
Year : 2017
Volume : 80
Issue : 3
First Page : 659
Last Page : 669
Authors : Chen WL, Ren Y, Ren J, Erxleben C, Johnson ME, Gentile S, Kinghorn AD, Swanson SM, Burdette JE.
Abstract : (+)-Strebloside, a cardiac glycoside isolated from the stem bark of Streblus asper collected in Vietnam, has shown some potential for further investigation as an antineoplastic agent. A mechanistic study using an in vitro assay and molecular docking analysis indicated that (+)-strebloside binds and inhibits Na+/K+-ATPase in a similar manner to digitoxin. Inhibition of growth of different high-grade serous ovarian cancer cells including OVCAR3, OVSAHO, Kuramochi, OVCAR4, OVCAR5, and OVCAR8 resulted from treatment with (+)-strebloside. Furthermore, this compound blocked cell cycle progression at the G2 phase and induced PARP cleavage, indicating apoptosis activation in OVCAR3 cells. (+)-Strebloside potently inhibited mutant p53 expression through the induction of ERK pathways and inhibited NF-κB activity in human ovarian cancer cells. However, in spite of its antitumor potential, the overall biological activity of (+)-strebloside must be regarded as being typical of better-known cardiac glycosides such as digoxin and ouabain. Further chemical alteration of cardiac glycosides might help to reduce negative side effects while increasing cancer cell cytotoxicity.
|
Antiproliferative activity against human OVSAHO cells after 72 hrs by CellTiter 96 aqueous one solution assay
|
Homo sapiens
|
250.2
nM
|
|
Journal : J Nat Prod
Title : (+)-Strebloside-Induced Cytotoxicity in Ovarian Cancer Cells Is Mediated through Cardiac Glycoside Signaling Networks.
Year : 2017
Volume : 80
Issue : 3
First Page : 659
Last Page : 669
Authors : Chen WL, Ren Y, Ren J, Erxleben C, Johnson ME, Gentile S, Kinghorn AD, Swanson SM, Burdette JE.
Abstract : (+)-Strebloside, a cardiac glycoside isolated from the stem bark of Streblus asper collected in Vietnam, has shown some potential for further investigation as an antineoplastic agent. A mechanistic study using an in vitro assay and molecular docking analysis indicated that (+)-strebloside binds and inhibits Na+/K+-ATPase in a similar manner to digitoxin. Inhibition of growth of different high-grade serous ovarian cancer cells including OVCAR3, OVSAHO, Kuramochi, OVCAR4, OVCAR5, and OVCAR8 resulted from treatment with (+)-strebloside. Furthermore, this compound blocked cell cycle progression at the G2 phase and induced PARP cleavage, indicating apoptosis activation in OVCAR3 cells. (+)-Strebloside potently inhibited mutant p53 expression through the induction of ERK pathways and inhibited NF-κB activity in human ovarian cancer cells. However, in spite of its antitumor potential, the overall biological activity of (+)-strebloside must be regarded as being typical of better-known cardiac glycosides such as digoxin and ouabain. Further chemical alteration of cardiac glycosides might help to reduce negative side effects while increasing cancer cell cytotoxicity.
|
Inhibition of sodium potassium ATPase in human OVCAR3 cells assessed as reduction in TNFalpha-induced NFkappaB activation at 100 nM after 4 hrs by luciferase reporter gene assay relative to control
|
Homo sapiens
|
50.0
%
|
|
Journal : J Nat Prod
Title : (+)-Strebloside-Induced Cytotoxicity in Ovarian Cancer Cells Is Mediated through Cardiac Glycoside Signaling Networks.
Year : 2017
Volume : 80
Issue : 3
First Page : 659
Last Page : 669
Authors : Chen WL, Ren Y, Ren J, Erxleben C, Johnson ME, Gentile S, Kinghorn AD, Swanson SM, Burdette JE.
Abstract : (+)-Strebloside, a cardiac glycoside isolated from the stem bark of Streblus asper collected in Vietnam, has shown some potential for further investigation as an antineoplastic agent. A mechanistic study using an in vitro assay and molecular docking analysis indicated that (+)-strebloside binds and inhibits Na+/K+-ATPase in a similar manner to digitoxin. Inhibition of growth of different high-grade serous ovarian cancer cells including OVCAR3, OVSAHO, Kuramochi, OVCAR4, OVCAR5, and OVCAR8 resulted from treatment with (+)-strebloside. Furthermore, this compound blocked cell cycle progression at the G2 phase and induced PARP cleavage, indicating apoptosis activation in OVCAR3 cells. (+)-Strebloside potently inhibited mutant p53 expression through the induction of ERK pathways and inhibited NF-κB activity in human ovarian cancer cells. However, in spite of its antitumor potential, the overall biological activity of (+)-strebloside must be regarded as being typical of better-known cardiac glycosides such as digoxin and ouabain. Further chemical alteration of cardiac glycosides might help to reduce negative side effects while increasing cancer cell cytotoxicity.
|
Antiviral activity against SARS-CoV-2 (viral titer) measured by plaque assay in Vero cells at MOI 0.0125 after 24 hr
|
Chlorocebus sabaeus
|
230.0
nM
|
|
Title : Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs
Year : 2020
Authors : Sangeun Jeon, Meehyun Ko, Jihye Lee, Inhee Choi, Soo Young Byun, Soonju Park, David Shum, Seungtaek Kim
Abstract : COVID-19 is an emerging infectious disease and was recently declared as a pandemic by WHO. Currently, there is no vaccine or therapeutic available for this disease. Drug repositioning represents the only feasible option to address this global challenge and a panel of 48 FDA- approved drugs that have been pre-selected by an assay of SARS-CoV was screened to identify potential antiviral drug candidates against SARS-CoV-2 infection. We found a total of 24 drugs which exhibited antiviral efficacy (0.1 μM < IC50 < 10 μM) against SARS-CoV-2. In particular, two FDA-approved drugs - niclosamide and ciclesonide – were notable in some respects. These drugs will be tested in an appropriate animal model for their antiviral activities. In near future, these already FDA-approved drugs could be further developed following clinical trials in order to provide additional therapeutic options for patients with COVID-19.
|
SARS-CoV-2 3CL-Pro protease inhibition percentage at 20µM by FRET kind of response from peptide substrate
|
Severe acute respiratory syndrome coronavirus 2
|
16.02
%
|
|
SARS-CoV-2 3CL-Pro protease inhibition percentage at 20µM by FRET kind of response from peptide substrate
|
Severe acute respiratory syndrome coronavirus 2
|
23.91
%
|
|
Title : Identification of inhibitors of SARS-Cov2 M-Pro enzymatic activity using a small molecule repurposing screen
Year : 2020
Authors : Maria Kuzikov, Elisa Costanzi, Jeanette Reinshagen, Francesca Esposito, Laura Vangeel, Markus Wolf, Bernhard Ellinger, Carsten Claussen, Gerd Geisslinger, Angela Corona, Daniela Iaconis, Carmine Talarico, Candida Manelfi, Rolando Cannalire, Giulia Rossetti, Jonas Gossen, Simone Albani, Francesco Musiani, Katja Herzog, Yang Ye, Barbara Giabbai, Nicola Demitri, Dirk Jochmans, Steven De Jonghe, Jasper Rymenants, Vincenzo Summa, Enzo Tramontano, Andrea R. Beccari, Pieter Leyssen, Paola Storici, Johan Neyts, Philip Gribbon, and Andrea Zaliani
Abstract : Compound repurposing is an important strategy being pursued in the identification of effective treatment against the SARS-CoV-2 infection and COVID-19 disease. In this regard, SARS-CoV-2 main protease (M-Pro), also termed 3CL-Pro, is an attractive drug target as it plays a central role in viral replication by processing the viral polyprotein into 11 non-structural proteins. We report the results of a screening campaign involving ca 8.7 K compounds containing marketed drugs, clinical and preclinical candidates, and chemicals regarded as safe in humans. We confirmed previously reported inhibitors of 3CL-Pro, but we have also identified 68 compounds with IC50 lower than 1 uM and 127 compounds with IC50 lower than 5 uM. Profiling showed 67% of confirmed hits were selective (> 5 fold) against other Cys- and Ser- proteases (Chymotrypsin and Cathepsin-L) and MERS 3CL-Pro. Selected compounds were also analysed in their binding characteristics.
|
Antiviral activity determined as inhibition of SARS-CoV-2 induced cytotoxicity of VERO-6 cells at 10 uM after 48 hours exposure to 0.01 MOI SARS CoV-2 virus by high content imaging
|
Chlorocebus sabaeus
|
-0.28
%
|
|
Antiviral activity determined as inhibition of SARS-CoV-2 induced cytotoxicity of VERO-6 cells at 10 uM after 48 hours exposure to 0.01 MOI SARS CoV-2 virus by high content imaging
|
Chlorocebus sabaeus
|
-0.28
%
|
|
Antiviral activity determined as inhibition of SARS-CoV-2 induced cytotoxicity of VERO-6 cells at 10 uM after 48 hours exposure to 0.01 MOI SARS CoV-2 virus by high content imaging
|
Chlorocebus sabaeus
|
-0.28
%
|
|
Antiviral activity determined as inhibition of SARS-CoV-2 induced cytotoxicity of VERO-6 cells at 10 uM after 48 hours exposure to 0.01 MOI SARS CoV-2 virus by high content imaging
|
Chlorocebus sabaeus
|
-0.28
%
|
|
Title : Cytopathic SARS-Cov2 screening on VERO-E6 cells in a large repurposing effort
Year : 2020
Authors : Andrea Zaliani, Laura Vangeel, Jeanette Reinshagen, Daniela Iaconis, Maria Kuzikov, Oliver Keminer, Markus Wolf, Bernhard Ellinger, Francesca Esposito, Angela Corona, Enzo Tramontano, Candida Manelfi, Katja Herzog, Dirk Jochmans, Steven De Jonghe, Winston Chiu, Thibault Francken, Joost Schepers, Caroline Collard, Kayvan Abbasi, Carsten Claussen , Vincenzo Summa, Andrea R. Beccari, Johan Neyts, Philip Gribbon and Pieter Leyssen
Abstract : Worldwide, there are intensive efforts to identify repurposed drugs as potential therapies against SARS-CoV-2 infection and the associated COVID-19 disease. To date, the anti-inflammatory drug dexamethasone and (to a lesser extent) the RNA-polymerase inhibitor remdesivir have been shown to be effective in reducing mortality and patient time to recovery, respectively, in patients. Here, we report the results of a phenotypic screening campaign within an EU-funded project (H2020-EXSCALATE4COV) aimed at extending the repertoire of anti-COVID therapeutics through repurposing of available compounds and highlighting compounds with new mechanisms of action against viral infection. We screened 8702 molecules from different repurposing libraries, to reveal 110 compounds with an anti-cytopathic IC50 < 20 µM. From this group, 18 with a safety index greater than 2 are also marketed drugs, making them suitable for further study as potential therapies against COVID-19. Our result supports the idea that a systematic approach to repurposing is a valid strategy to accelerate the necessary drug discovery process.
|
Inhibition of Sodium/potassium-transporting ATPase in porcine cerebral cortex using ATP as substrate incubated for 15 mins by ADP-Glo Max Assay
|
Sus scrofa
|
126.8
nM
|
|
Journal : Bioorg Med Chem
Title : Cytotoxic and non-cytotoxic cardiac glycosides isolated from the combined flowers, leaves, and twigs of Streblus asper.
Year : 2020
Volume : 28
Issue : 4
First Page : 115301
Last Page : 115301
Authors : Ren Y, Tan Q, Heath K, Wu S, Wilson JR, Ren J, Shriwas P, Yuan C, Ngoc Ninh T, Chai HB, Chen X, Soejarto DD, Johnson ME, Cheng X, Burdette JE, Kinghorn AD.
Abstract : A new non-cytotoxic [(+)-17β-hydroxystrebloside (1)] and two known cytotoxic [(+)-3'-de-O-methylkamaloside (2) and (+)-strebloside (3)] cardiac glycosides were isolated and identified from the combined flowers, leaves, and twigs of Streblus asper collected in Vietnam, with the absolute configuration of 1 established from analysis of its ECD and NMR spectroscopic data and confirmed by computational ECD calculations. A new 14,21-epoxycardanolide (3a) was synthesized from 3 that was treated with base. A preliminary structure-activity relationship study indicated that the C-14 hydroxy group and the C-17 lactone unit and the established conformation are important for the mediation of the cytotoxicity of 3. Molecular docking profiles showed that the cytotoxic 3 and its non-cytotoxic analogue 1 bind differentially to Na<sup>+</sup>/K<sup>+</sup>-ATPase. Compound 3 docks deeply in the Na<sup>+</sup>/K<sup>+</sup>-ATPase pocket with a sole pose, and its C-10 formyl and C-5, C-14, and C-4' hydroxy groups may form hydrogen bonds with the side-chains of Glu111, Glu117, Thr797, and Arg880 of Na<sup>+</sup>/K<sup>+</sup>-ATPase, respectively. However, 1 fits the cation binding sites with at least three different poses, which all depotentiate the binding between 1 and Na<sup>+</sup>/K<sup>+</sup>-ATPase. Thus, 3 was found to inhibit Na<sup>+</sup>/K<sup>+</sup>-ATPase, but 1 did not. In addition, the cytotoxic and Na<sup>+</sup>/K<sup>+</sup>-ATPase inhibitory 3 did not affect glucose uptake in human lung cancer cells, against which it showed potent activity, indicating that this cardiac glycoside mediates its cytotoxicity by targeting Na<sup>+</sup>/K<sup>+</sup>-ATPase but not by interacting with glucose transporters.
|
Cytotoxicity against human HT-29 cells after 72 hrs by CellTiter 96 Aqueous One Solution reagent based assay
|
Homo sapiens
|
68.0
nM
|
|
Journal : J Nat Prod
Title : Na<sup>+</sup>/K<sup>+</sup>-ATPase-Targeted Cytotoxicity of (+)-Digoxin and Several Semisynthetic Derivatives.
Year : 2020
Volume : 83
Issue : 3
First Page : 638
Last Page : 648
Authors : Ren Y, Ribas HT, Heath K, Wu S, Ren J, Shriwas P, Chen X, Johnson ME, Cheng X, Burdette JE, Kinghorn AD.
Abstract : (+)-Digoxin (<b>1</b>) is a well-known cardiac glycoside long used to treat congestive heart failure and found more recently to show anticancer activity. Several known cardenolides (<b>2</b>-<b>5</b>) and two new analogues, (+)-8(9)-β-anhydrodigoxigenin (<b>6</b>) and (+)-17-<i>epi</i>-20,22-dihydro-21α-hydroxydigoxin (<b>7</b>), were synthesized from <b>1</b> and evaluated for their cytotoxicity toward a small panel of human cancer cell lines. A preliminary structure-activity relationship investigation conducted indicated that the C-12 and C-14 hydroxy groups and the C-17 unsaturated lactone unit are important for <b>1</b> to mediate its cytotoxicity toward human cancer cells, but the C-3 glycosyl residue seems to be less critical for such an effect. Molecular docking profiles showed that the cytotoxic <b>1</b> and the noncytotoxic derivative <b>7</b> bind differentially to Na<sup>+</sup>/K<sup>+</sup>-ATPase. The HO-12β, HO-14β, and HO-3'aα hydroxy groups of (+)-digoxin (<b>1</b>) may form hydrogen bonds with the side-chains of Asp121 and Asn122, Thr797, and Arg880 of Na<sup>+</sup>/K<sup>+</sup>-ATPase, respectively, but the altered lactone unit of <b>7</b> results in a rotation of its steroid core, which depotentiates the binding between this compound and Na<sup>+</sup>/K<sup>+</sup>-ATPase. Thus, <b>1</b> was found to inhibit Na<sup>+</sup>/K<sup>+</sup>-ATPase, but <b>7</b> did not. In addition, the cytotoxic <b>1</b> did not affect glucose uptake in human cancer cells, indicating that this cardiac glycoside mediates its cytotoxicity by targeting Na<sup>+</sup>/K<sup>+</sup>-ATPase but not by interacting with glucose transporters.
|
Cytotoxicity against human MDA-MB-231 cells after 72 hrs by CellTiter 96 Aqueous One Solution reagent based assay
|
Homo sapiens
|
480.0
nM
|
|
Journal : J Nat Prod
Title : Na<sup>+</sup>/K<sup>+</sup>-ATPase-Targeted Cytotoxicity of (+)-Digoxin and Several Semisynthetic Derivatives.
Year : 2020
Volume : 83
Issue : 3
First Page : 638
Last Page : 648
Authors : Ren Y, Ribas HT, Heath K, Wu S, Ren J, Shriwas P, Chen X, Johnson ME, Cheng X, Burdette JE, Kinghorn AD.
Abstract : (+)-Digoxin (<b>1</b>) is a well-known cardiac glycoside long used to treat congestive heart failure and found more recently to show anticancer activity. Several known cardenolides (<b>2</b>-<b>5</b>) and two new analogues, (+)-8(9)-β-anhydrodigoxigenin (<b>6</b>) and (+)-17-<i>epi</i>-20,22-dihydro-21α-hydroxydigoxin (<b>7</b>), were synthesized from <b>1</b> and evaluated for their cytotoxicity toward a small panel of human cancer cell lines. A preliminary structure-activity relationship investigation conducted indicated that the C-12 and C-14 hydroxy groups and the C-17 unsaturated lactone unit are important for <b>1</b> to mediate its cytotoxicity toward human cancer cells, but the C-3 glycosyl residue seems to be less critical for such an effect. Molecular docking profiles showed that the cytotoxic <b>1</b> and the noncytotoxic derivative <b>7</b> bind differentially to Na<sup>+</sup>/K<sup>+</sup>-ATPase. The HO-12β, HO-14β, and HO-3'aα hydroxy groups of (+)-digoxin (<b>1</b>) may form hydrogen bonds with the side-chains of Asp121 and Asn122, Thr797, and Arg880 of Na<sup>+</sup>/K<sup>+</sup>-ATPase, respectively, but the altered lactone unit of <b>7</b> results in a rotation of its steroid core, which depotentiates the binding between this compound and Na<sup>+</sup>/K<sup>+</sup>-ATPase. Thus, <b>1</b> was found to inhibit Na<sup>+</sup>/K<sup>+</sup>-ATPase, but <b>7</b> did not. In addition, the cytotoxic <b>1</b> did not affect glucose uptake in human cancer cells, indicating that this cardiac glycoside mediates its cytotoxicity by targeting Na<sup>+</sup>/K<sup>+</sup>-ATPase but not by interacting with glucose transporters.
|
Cytotoxicity against human OVCAR3 cells after 72 hrs by CellTiter 96 Aqueous One Solution reagent based assay
|
Homo sapiens
|
120.0
nM
|
|
Journal : J Nat Prod
Title : Na<sup>+</sup>/K<sup>+</sup>-ATPase-Targeted Cytotoxicity of (+)-Digoxin and Several Semisynthetic Derivatives.
Year : 2020
Volume : 83
Issue : 3
First Page : 638
Last Page : 648
Authors : Ren Y, Ribas HT, Heath K, Wu S, Ren J, Shriwas P, Chen X, Johnson ME, Cheng X, Burdette JE, Kinghorn AD.
Abstract : (+)-Digoxin (<b>1</b>) is a well-known cardiac glycoside long used to treat congestive heart failure and found more recently to show anticancer activity. Several known cardenolides (<b>2</b>-<b>5</b>) and two new analogues, (+)-8(9)-β-anhydrodigoxigenin (<b>6</b>) and (+)-17-<i>epi</i>-20,22-dihydro-21α-hydroxydigoxin (<b>7</b>), were synthesized from <b>1</b> and evaluated for their cytotoxicity toward a small panel of human cancer cell lines. A preliminary structure-activity relationship investigation conducted indicated that the C-12 and C-14 hydroxy groups and the C-17 unsaturated lactone unit are important for <b>1</b> to mediate its cytotoxicity toward human cancer cells, but the C-3 glycosyl residue seems to be less critical for such an effect. Molecular docking profiles showed that the cytotoxic <b>1</b> and the noncytotoxic derivative <b>7</b> bind differentially to Na<sup>+</sup>/K<sup>+</sup>-ATPase. The HO-12β, HO-14β, and HO-3'aα hydroxy groups of (+)-digoxin (<b>1</b>) may form hydrogen bonds with the side-chains of Asp121 and Asn122, Thr797, and Arg880 of Na<sup>+</sup>/K<sup>+</sup>-ATPase, respectively, but the altered lactone unit of <b>7</b> results in a rotation of its steroid core, which depotentiates the binding between this compound and Na<sup>+</sup>/K<sup>+</sup>-ATPase. Thus, <b>1</b> was found to inhibit Na<sup>+</sup>/K<sup>+</sup>-ATPase, but <b>7</b> did not. In addition, the cytotoxic <b>1</b> did not affect glucose uptake in human cancer cells, indicating that this cardiac glycoside mediates its cytotoxicity by targeting Na<sup>+</sup>/K<sup>+</sup>-ATPase but not by interacting with glucose transporters.
|
Cytotoxicity against human MDA-MB-435 cells after 72 hrs by CellTiter 96 Aqueous One Solution reagent based assay
|
Homo sapiens
|
43.0
nM
|
|
Journal : J Nat Prod
Title : Na<sup>+</sup>/K<sup>+</sup>-ATPase-Targeted Cytotoxicity of (+)-Digoxin and Several Semisynthetic Derivatives.
Year : 2020
Volume : 83
Issue : 3
First Page : 638
Last Page : 648
Authors : Ren Y, Ribas HT, Heath K, Wu S, Ren J, Shriwas P, Chen X, Johnson ME, Cheng X, Burdette JE, Kinghorn AD.
Abstract : (+)-Digoxin (<b>1</b>) is a well-known cardiac glycoside long used to treat congestive heart failure and found more recently to show anticancer activity. Several known cardenolides (<b>2</b>-<b>5</b>) and two new analogues, (+)-8(9)-β-anhydrodigoxigenin (<b>6</b>) and (+)-17-<i>epi</i>-20,22-dihydro-21α-hydroxydigoxin (<b>7</b>), were synthesized from <b>1</b> and evaluated for their cytotoxicity toward a small panel of human cancer cell lines. A preliminary structure-activity relationship investigation conducted indicated that the C-12 and C-14 hydroxy groups and the C-17 unsaturated lactone unit are important for <b>1</b> to mediate its cytotoxicity toward human cancer cells, but the C-3 glycosyl residue seems to be less critical for such an effect. Molecular docking profiles showed that the cytotoxic <b>1</b> and the noncytotoxic derivative <b>7</b> bind differentially to Na<sup>+</sup>/K<sup>+</sup>-ATPase. The HO-12β, HO-14β, and HO-3'aα hydroxy groups of (+)-digoxin (<b>1</b>) may form hydrogen bonds with the side-chains of Asp121 and Asn122, Thr797, and Arg880 of Na<sup>+</sup>/K<sup>+</sup>-ATPase, respectively, but the altered lactone unit of <b>7</b> results in a rotation of its steroid core, which depotentiates the binding between this compound and Na<sup>+</sup>/K<sup>+</sup>-ATPase. Thus, <b>1</b> was found to inhibit Na<sup>+</sup>/K<sup>+</sup>-ATPase, but <b>7</b> did not. In addition, the cytotoxic <b>1</b> did not affect glucose uptake in human cancer cells, indicating that this cardiac glycoside mediates its cytotoxicity by targeting Na<sup>+</sup>/K<sup>+</sup>-ATPase but not by interacting with glucose transporters.