Structure
| InChI Key | UREBDLICKHMUKA-CXSFZGCWSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C22H29FO5 |
| Molecular Weight | 392.47 |
| AlogP | 1.9 |
| Hydrogen Bond Acceptor | 5.0 |
| Hydrogen Bond Donor | 3.0 |
| Number of Rotational Bond | 2.0 |
| Polar Surface Area | 94.83 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 0.0 |
| Heavy Atoms | 28.0 |
Pharmacology
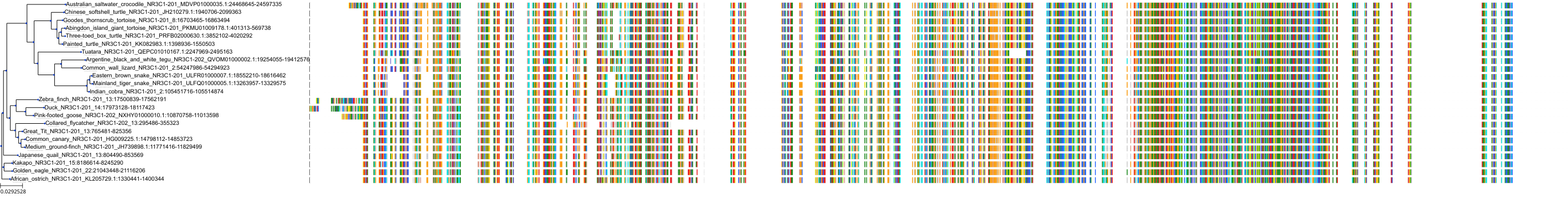
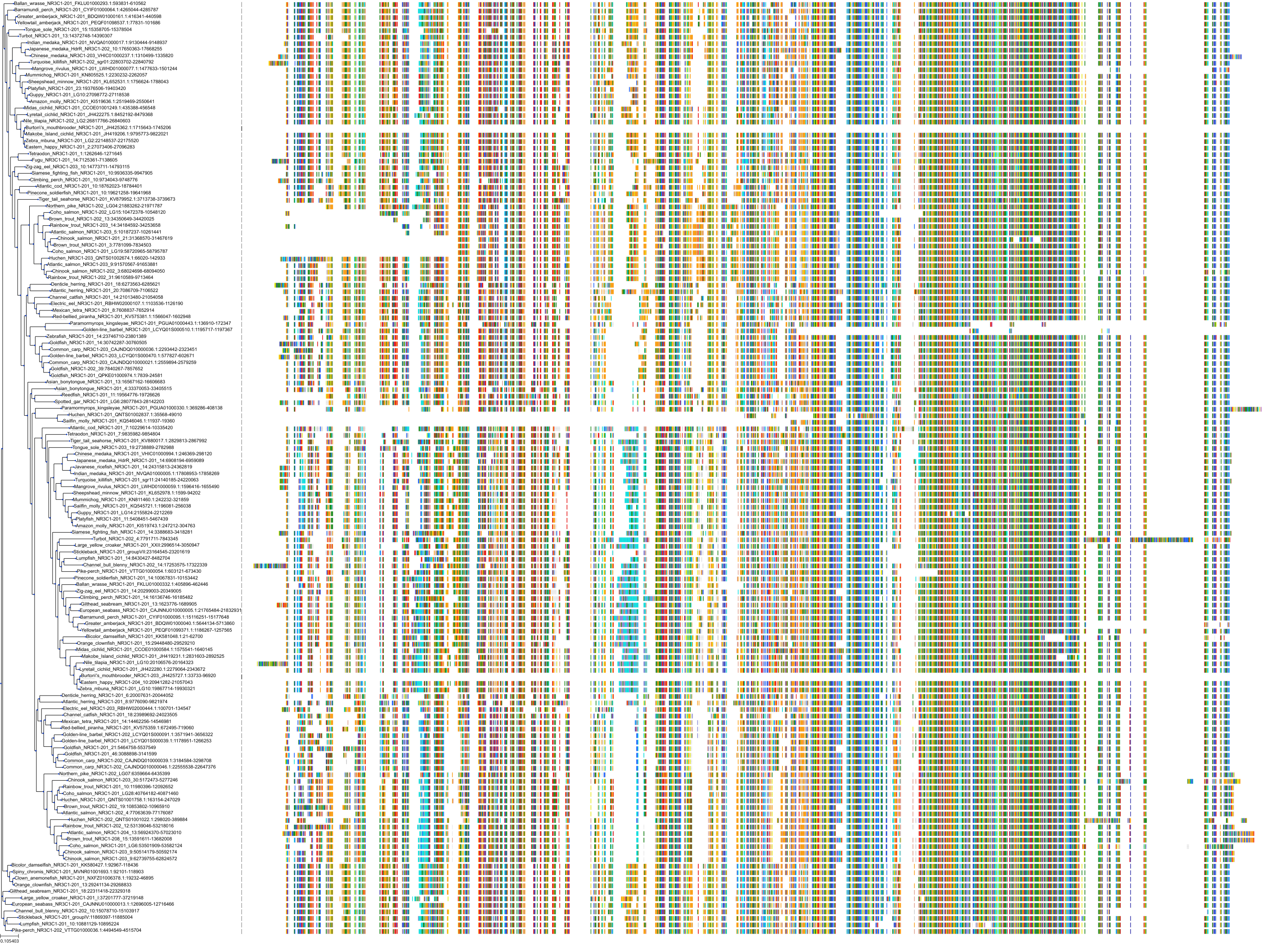
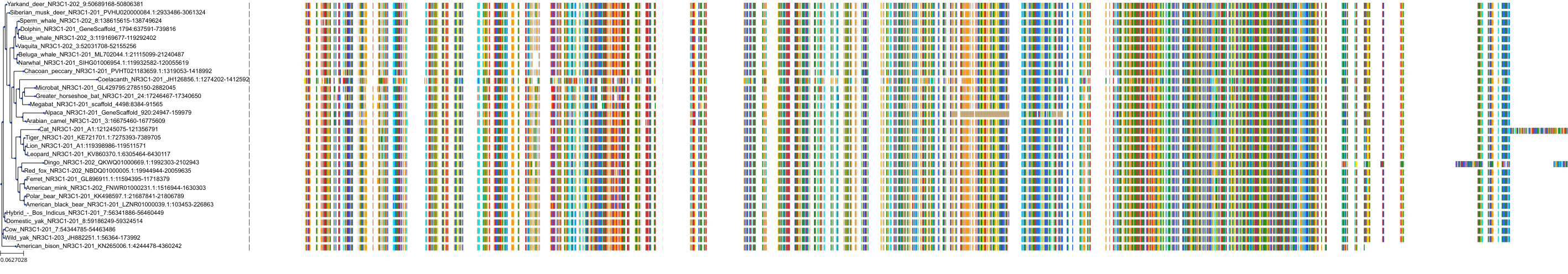
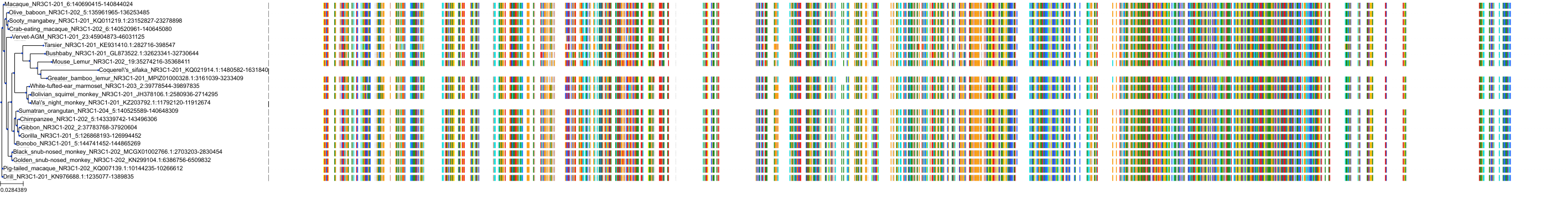
Target Conservation
|
Protein: Glucocorticoid receptor Description: Glucocorticoid receptor Organism : Homo sapiens P04150 ENSG00000113580 |
||||
Related Entries
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 41879 |
| ChEMBL | CHEMBL384467 |
| DrugBank | DB01234 |
| DrugCentral | 824 |
| FDA SRS | 7S5I7G3JQL |
| Human Metabolome Database | HMDB0015364 |
| Guide to Pharmacology | 3447 |
| KEGG | C15643 |
| PDB | DEX |
| PharmGKB | PA449247 |
| PubChem | 5743 |
| SureChEMBL | SCHEMBL3774 |
| ZINC | ZINC000003875332 |
 Cavia porcellus
Cavia porcellus
 Cricetulus griseus
Cricetulus griseus
 Homo sapiens
Homo sapiens
 Mus musculus
Mus musculus
 Rattus norvegicus
Rattus norvegicus
 Sus scrofa
Sus scrofa