| Trade Names | |
| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| ATC | A10BK01 |
| UNII | 1ULL0QJ8UC |
Structure
| InChI Key | JVHXJTBJCFBINQ-ADAARDCZSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C21H25ClO6 |
| Molecular Weight | 408.88 |
| AlogP | 1.84 |
| Hydrogen Bond Acceptor | 6.0 |
| Hydrogen Bond Donor | 4.0 |
| Number of Rotational Bond | 6.0 |
| Polar Surface Area | 99.38 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 2.0 |
| Heavy Atoms | 28.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Sodium/glucose cotransporter 2 inhibitor | INHIBITOR | DailyMed |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Transporter
Electrochemical transporter
SLC superfamily of solute carriers
SLC02 family of hexose and sugar alcohol transporters
|
- | - | - | - | 8-8 | |
|
Transporter
Electrochemical transporter
SLC superfamily of solute carriers
SLC05 family of sodium-dependent glucose transporters
|
1.1-860 | 0.49-891 | - | - | 69.7-103 |
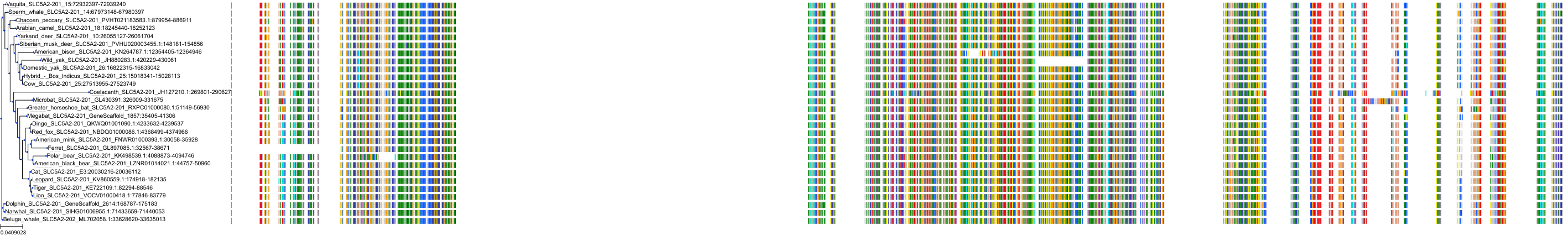
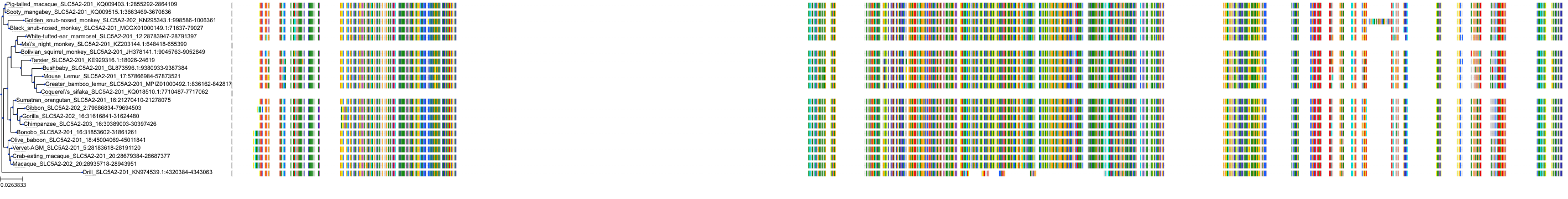
Target Conservation
|
Protein: Sodium/glucose cotransporter 2 Description: Sodium/glucose cotransporter 2 Organism : Homo sapiens P31639 ENSG00000140675 |
||||
Related Entries
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 85078 |
| ChEMBL | CHEMBL429910 |
| DrugBank | DB06292 |
| DrugCentral | 4304 |
| FDA SRS | 1ULL0QJ8UC |
| Guide to Pharmacology | 4594 |
| KEGG | D08897 |
| PubChem | 9887712 |
| SureChEMBL | SCHEMBL157820 |
| ZINC | ZINC000003819138 |
 Homo sapiens
Homo sapiens
 Rattus norvegicus
Rattus norvegicus