| Trade Names | |
| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| ATC | V03AX03 |
| UNII | LW2E03M5PG |
| EPA CompTox | DTXSID00143269 |
Structure
| InChI Key | ZCIGNRJZKPOIKD-CQXVEOKZSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C40H53N7O5S2 |
| Molecular Weight | 776.04 |
| AlogP | 6.0 |
| Hydrogen Bond Acceptor | 10.0 |
| Hydrogen Bond Donor | 3.0 |
| Number of Rotational Bond | 19.0 |
| Polar Surface Area | 138.02 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 4.0 |
| Heavy Atoms | 54.0 |
Pharmacology
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Enzyme
Cytochrome P450
Cytochrome P450 family 3
Cytochrome P450 family 3A
Cytochrome P450 3A4
|
- | 150 | - | - | - |
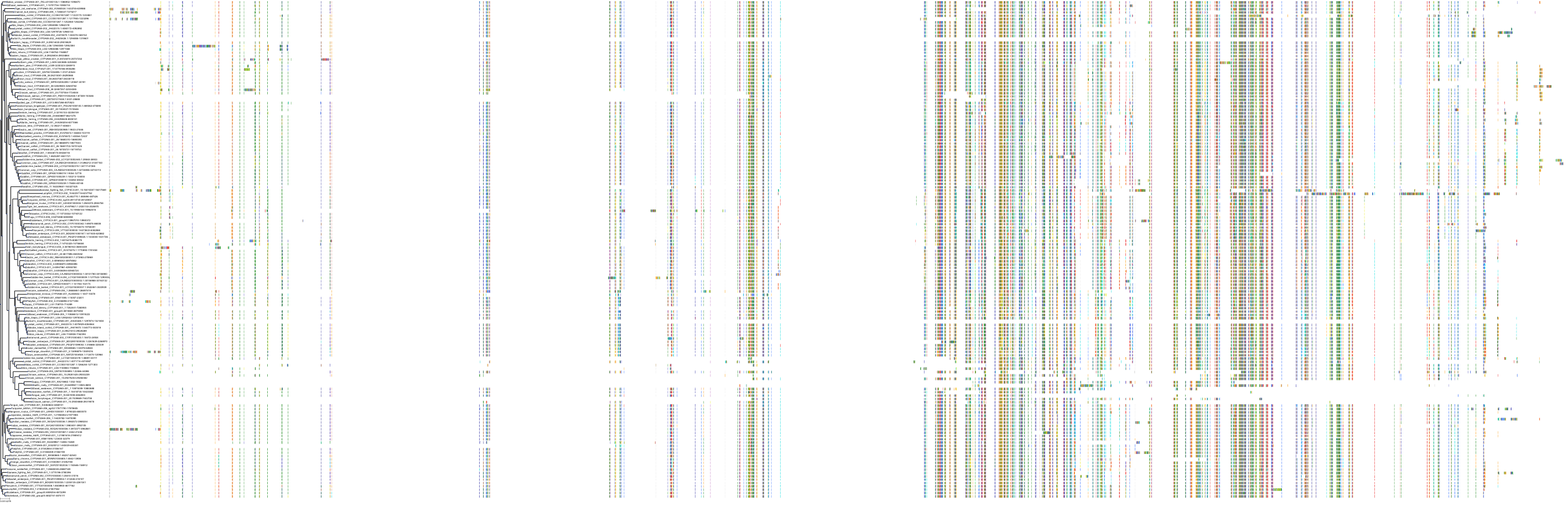
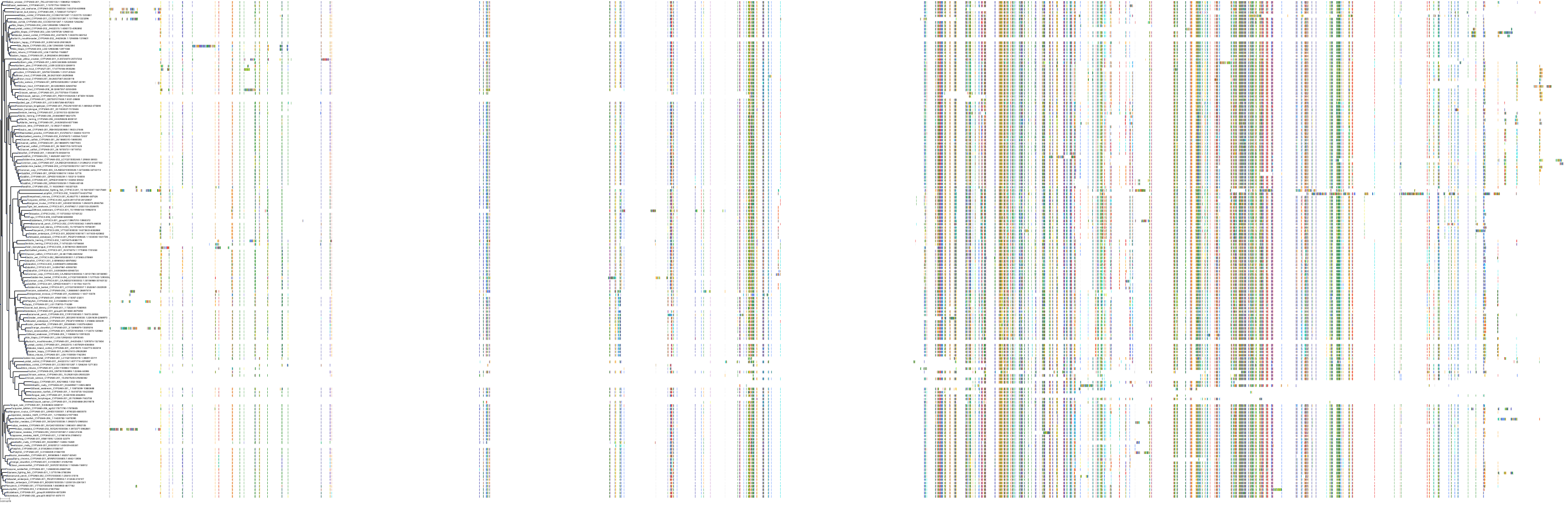
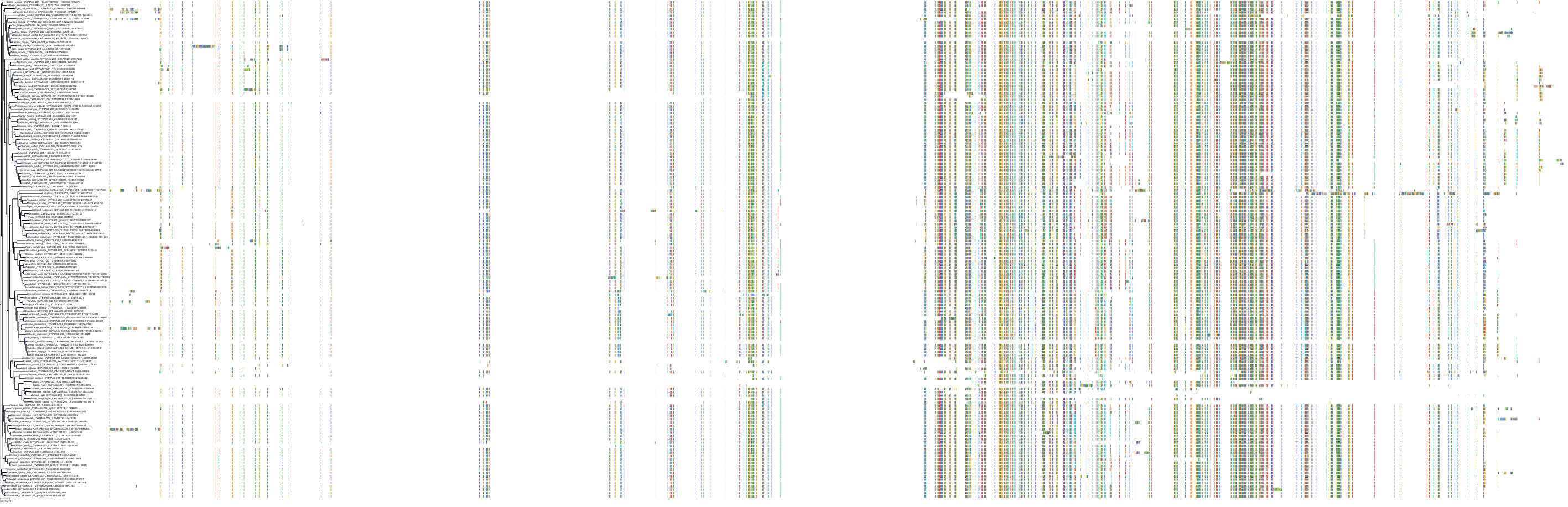
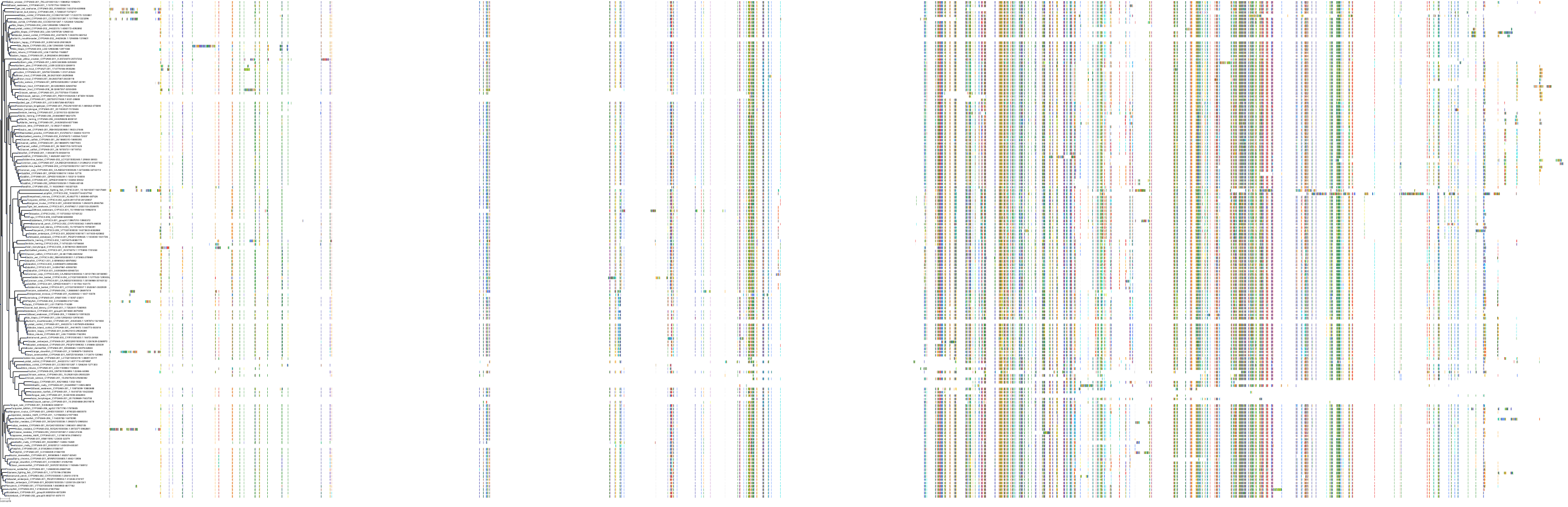
Target Conservation
|
Protein: Cytochrome P450 3A Description: Cytochrome P450 3A4 Organism : Homo sapiens P08684 ENSG00000160868 |
||||
|
Protein: Cytochrome P450 3A Description: Cytochrome P450 3A5 Organism : Homo sapiens P20815 ENSG00000106258 |
||||
|
Protein: Cytochrome P450 3A Description: Cytochrome P450 3A7 Organism : Homo sapiens P24462 ENSG00000160870 |
||||
|
Protein: Cytochrome P450 3A Description: Cytochrome P450 3A43 Organism : Homo sapiens Q9HB55 ENSG00000021461 |
||||
Related Entries
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 72291 |
| ChEMBL | CHEMBL2095208 |
| DrugBank | DB09065 |
| DrugCentral | 4299 |
| FDA SRS | LW2E03M5PG |
| Guide to Pharmacology | 7535 |
| PharmGKB | PA166165092 |
| PubChem | 25151504 |
| SureChEMBL | SCHEMBL2736227 |
| ZINC | ZINC000085537014 |
 Homo sapiens
Homo sapiens