| Trade Names | |
| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| UNII | EM5OCB9YJ6 |
| EPA CompTox | DTXSID3049043 |
| Parent Compound: | ABIRATERONE |
Structure
| InChI Key | UVIQSJCZCSLXRZ-UBUQANBQSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C26H33NO2 |
| Molecular Weight | 391.56 |
| AlogP | 5.97 |
| Hydrogen Bond Acceptor | 3.0 |
| Number of Rotational Bond | 2.0 |
| Polar Surface Area | 39.19 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 1.0 |
| Heavy Atoms | 29.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Cytochrome P450 17A1 inhibitor | INHIBITOR | FDA |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Enzyme
Cytochrome P450
Cytochrome P450 family 17
Cytochrome P450 family 17A
Cytochrome P450 17A1
|
- | 17-18 | - | - | - |
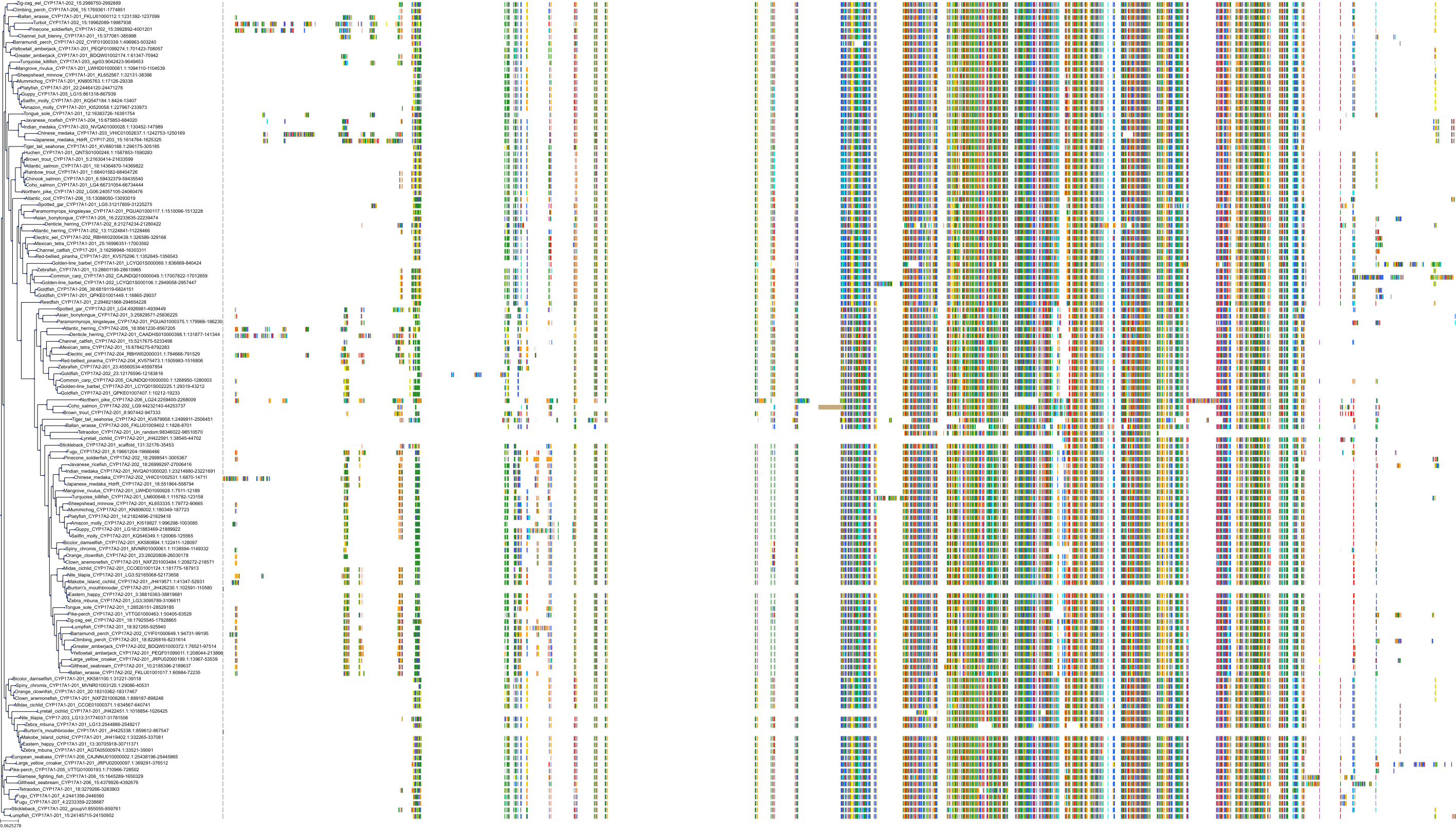
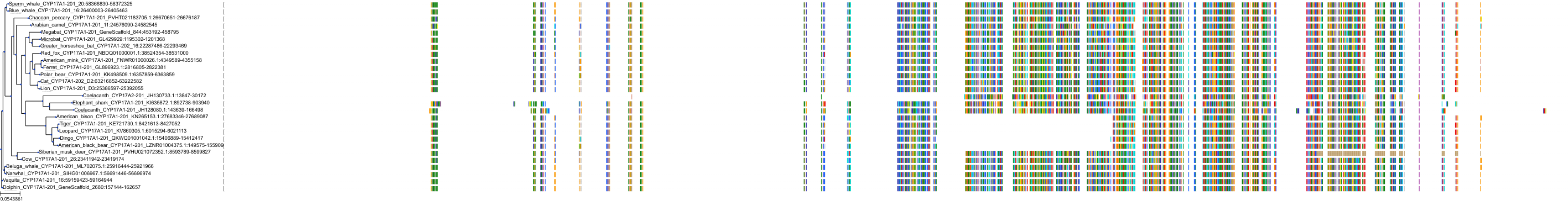
Target Conservation
|
Protein: Cytochrome P450 17A1 Description: Steroid 17-alpha-hydroxylase/17,20 lyase Organism : Homo sapiens P05093 ENSG00000148795 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 68639 |
| ChEMBL | CHEMBL271227 |
| DrugCentral | 4176 |
| FDA SRS | EM5OCB9YJ6 |
| Guide to Pharmacology | 9288 |
| KEGG | D09701 |
| PubChem | 9821849 |
| SureChEMBL | SCHEMBL93715 |
| ZINC | ZINC000003809191 |