Structure
| InChI Key | FKSFKBQGSFSOSM-QFIPXVFZSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C31H38N6O2 |
| Molecular Weight | 526.69 |
| AlogP | 4.63 |
| Hydrogen Bond Acceptor | 6.0 |
| Hydrogen Bond Donor | 3.0 |
| Number of Rotational Bond | 7.0 |
| Polar Surface Area | 95.05 |
| Molecular species | BASE |
| Aromatic Rings | 4.0 |
| Heavy Atoms | 39.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Histone-lysine N-methyltransferase EZH2 inhibitor | INHIBITOR | Other Other |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Epigenetic regulator
Writer
Protein methyltransferase
|
- | 1-280 | - | 0.5-0.5 | - | |
|
Unclassified protein
|
- | 16 | - | - | - |
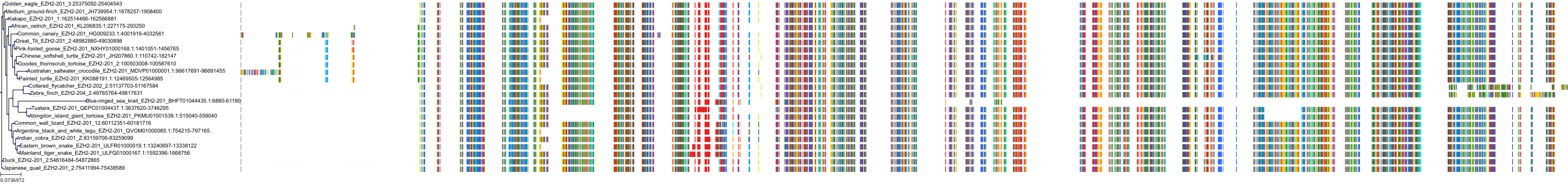
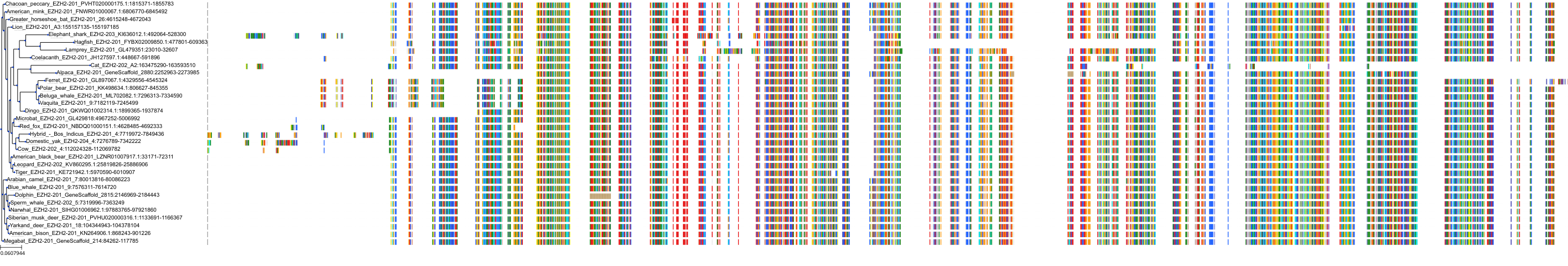
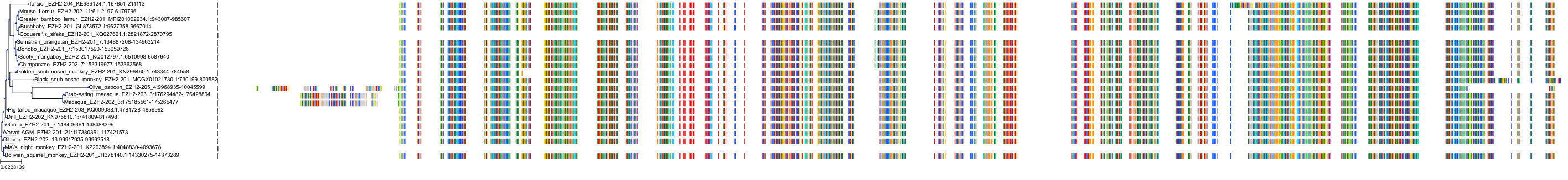
Target Conservation
|
Protein: Histone-lysine N-methyltransferase EZH2 Description: Histone-lysine N-methyltransferase EZH2 Organism : Homo sapiens Q15910 ENSG00000106462 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 124921 |
| ChEMBL | CHEMBL3287735 |
| FDA SRS | W4OGW9QZ97 |
| Guide to Pharmacology | 7012 |
| PDB | A9G |
| SureChEMBL | SCHEMBL12180401 |
| ZINC | ZINC000072318146 |
 Homo sapiens
Homo sapiens