| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| ATC | N02AX03 |
| UNII | VHX8K5SV4X |
| EPA CompTox | DTXSID2022911 |
Structure
| InChI Key | VTMVHDZWSFQSQP-VBNZEHGJSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C16H23NO |
| Molecular Weight | 245.37 |
| AlogP | 3.11 |
| Hydrogen Bond Acceptor | 2.0 |
| Hydrogen Bond Donor | 2.0 |
| Polar Surface Area | 46.25 |
| Molecular species | BASE |
| Aromatic Rings | 1.0 |
| Heavy Atoms | 18.0 |
Pharmacology
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Membrane receptor
Family A G protein-coupled receptor
Peptide receptor (family A GPCR)
Short peptide receptor (family A GPCR)
Opioid receptor
|
38 | - | - | - | - |
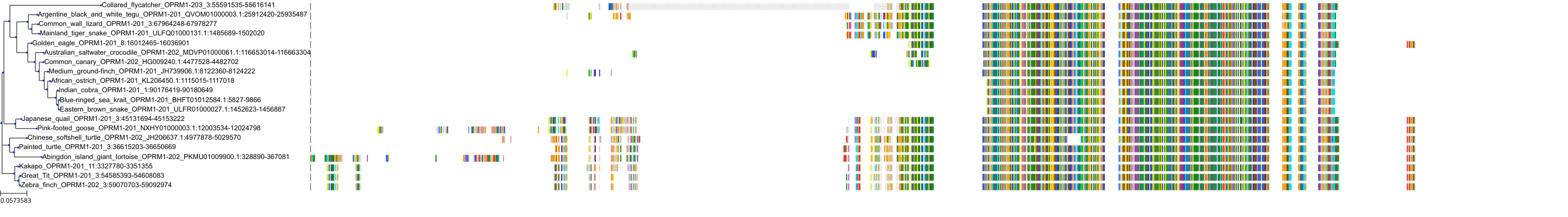
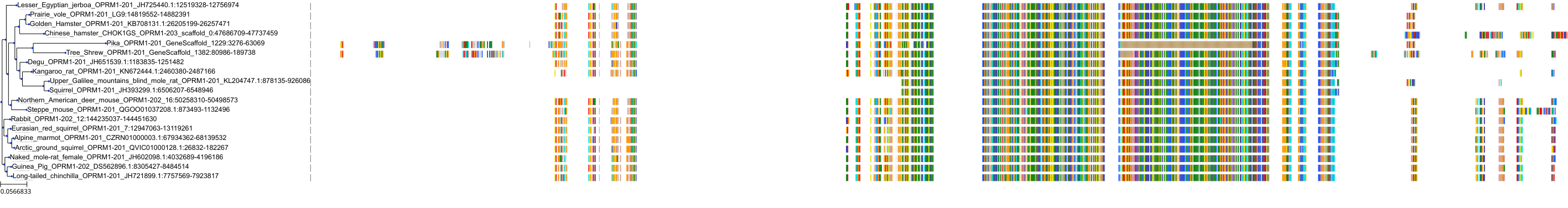
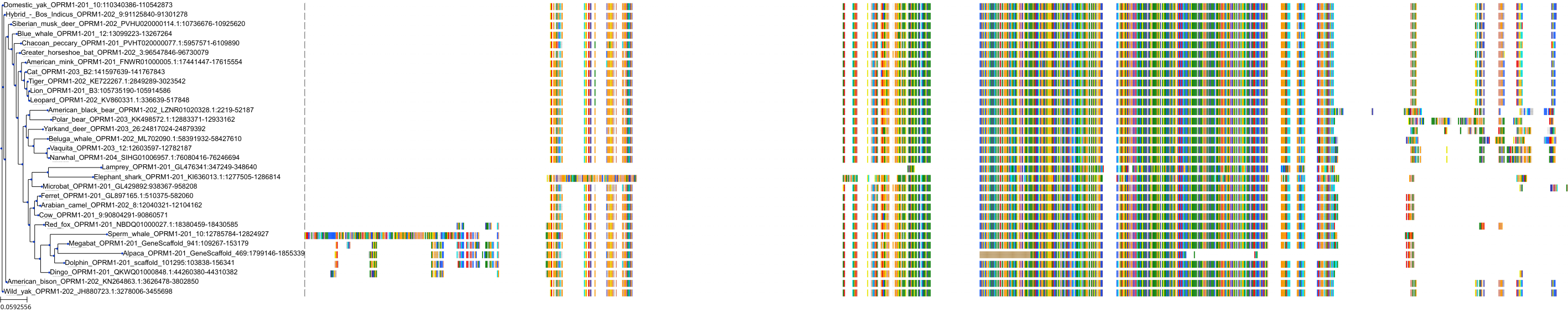
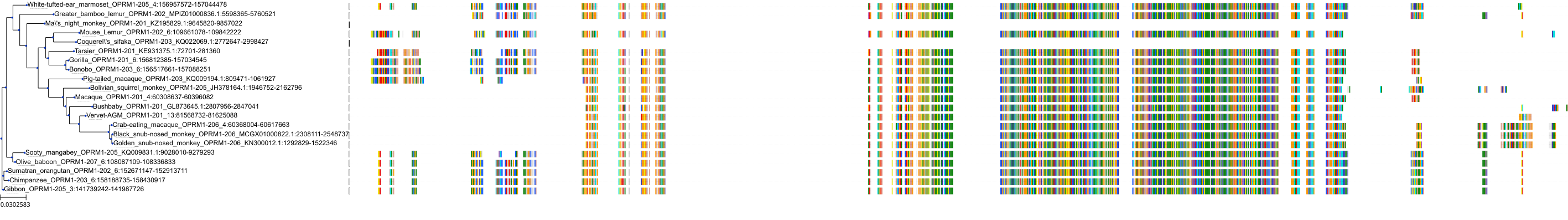
Target Conservation
|
Protein: Mu opioid receptor Description: Mu-type opioid receptor Organism : Homo sapiens P35372 ENSG00000112038 |
||||
|
Protein: Kappa opioid receptor Description: Kappa-type opioid receptor Organism : Homo sapiens P41145 ENSG00000082556 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 4474 |
| ChEMBL | CHEMBL1685 |
| DrugBank | DB01209 |
| DrugCentral | 847 |
| FDA SRS | VHX8K5SV4X |
| Human Metabolome Database | HMDB0015340 |
| SureChEMBL | SCHEMBL3072 |
 Homo sapiens
Homo sapiens