Structure
| InChI Key | NCRMKIWHFXSBGZ-CNBXIYLPSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C23H22Cl2F3NO3 |
| Molecular Weight | 488.33 |
| AlogP | 6.88 |
| Hydrogen Bond Acceptor | 2.0 |
| Hydrogen Bond Donor | 2.0 |
| Number of Rotational Bond | 8.0 |
| Polar Surface Area | 66.4 |
| Molecular species | ACID |
| Aromatic Rings | 2.0 |
| Heavy Atoms | 32.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Soluble guanylate cyclase activator | ACTIVATOR | PubMed |
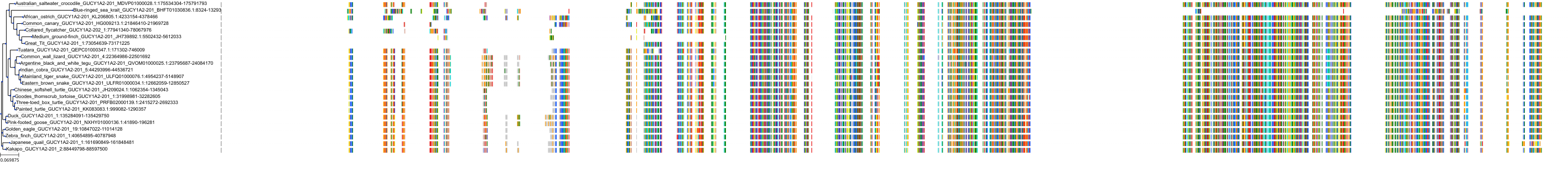
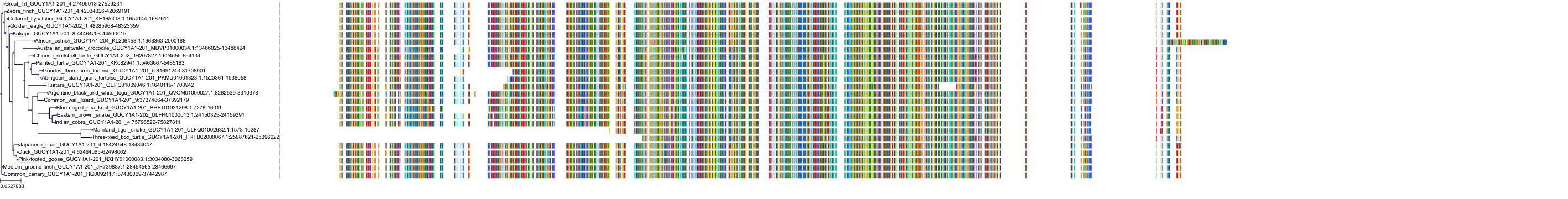
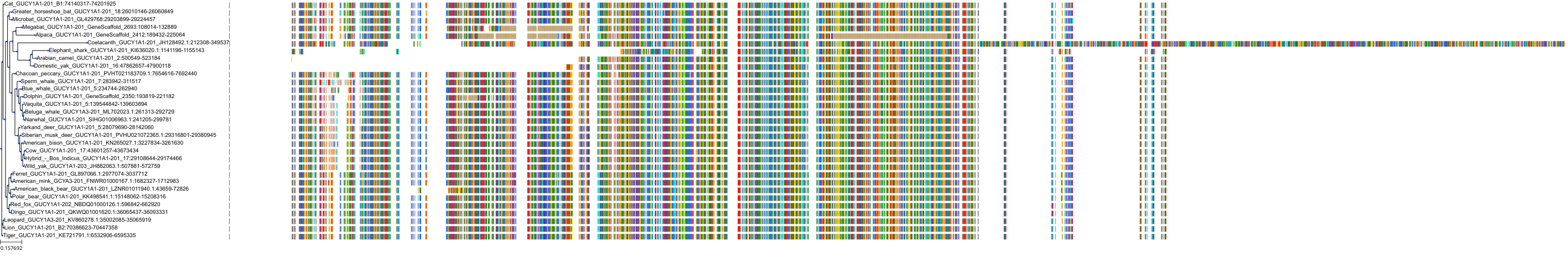
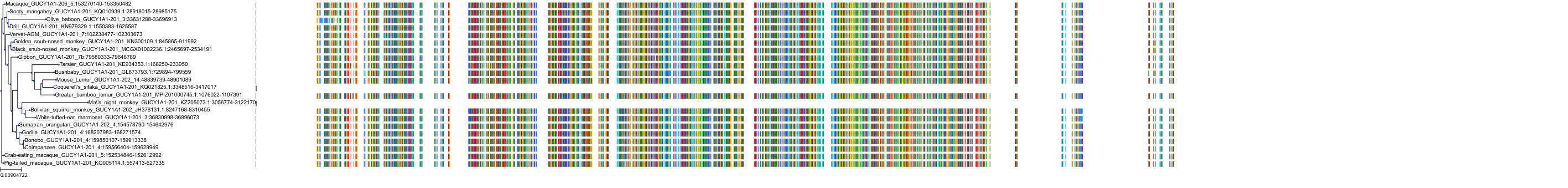
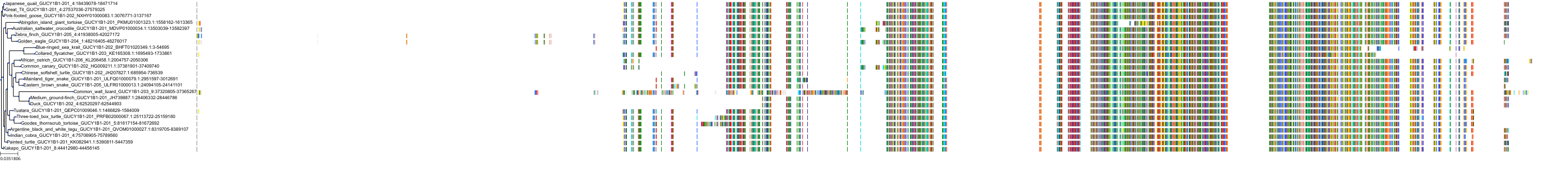
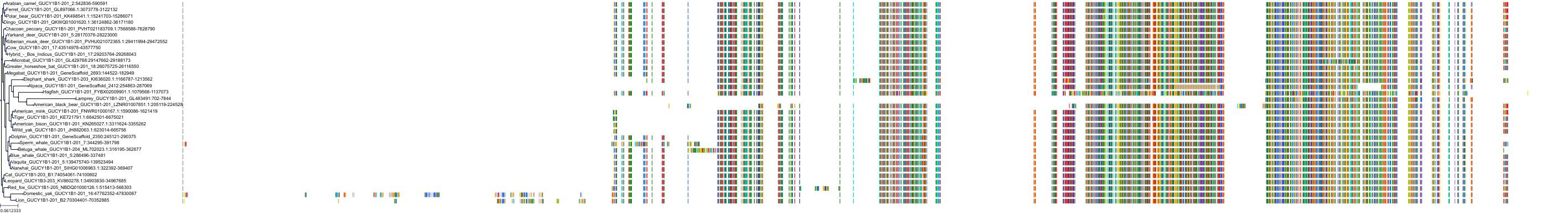
Target Conservation
|
Protein: Soluble guanylate cyclase Description: Guanylate cyclase soluble subunit alpha-2 Organism : Homo sapiens P33402 ENSG00000152402 |
||||
|
Protein: Soluble guanylate cyclase Description: Guanylate cyclase soluble subunit alpha-1 Organism : Homo sapiens Q02108 ENSG00000164116 |
||||
|
Protein: Soluble guanylate cyclase Description: Guanylate cyclase soluble subunit beta-1 Organism : Homo sapiens Q02153 ENSG00000061918 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL4650322 |
| FDA SRS | 5EZ01YDT5S |
 Oryctolagus cuniculus
Oryctolagus cuniculus
 Rattus norvegicus
Rattus norvegicus
 Sus scrofa
Sus scrofa