| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| ATC | L02BG06 |
| UNII | NY22HMQ4BX |
| EPA CompTox | DTXSID5023037 |
Structure
| InChI Key | BFYIZQONLCFLEV-DAELLWKTSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C20H24O2 |
| Molecular Weight | 296.41 |
| AlogP | 4.03 |
| Hydrogen Bond Acceptor | 2.0 |
| Polar Surface Area | 34.14 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 0.0 |
| Heavy Atoms | 22.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Cytochrome P450 19A1 inhibitor | INHIBITOR | DailyMed |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Enzyme
Cytochrome P450
Cytochrome P450 family 19
Cytochrome P450 family 19A
Cytochrome P450 19A1
|
- | 23-900 | - | 10.2-26 | 95.01-99.62 | |
|
Enzyme
Cytochrome P450
Cytochrome P450 family 3
Cytochrome P450 family 3A
Cytochrome P450 3A4
|
- | - | - | - | 45-95 |
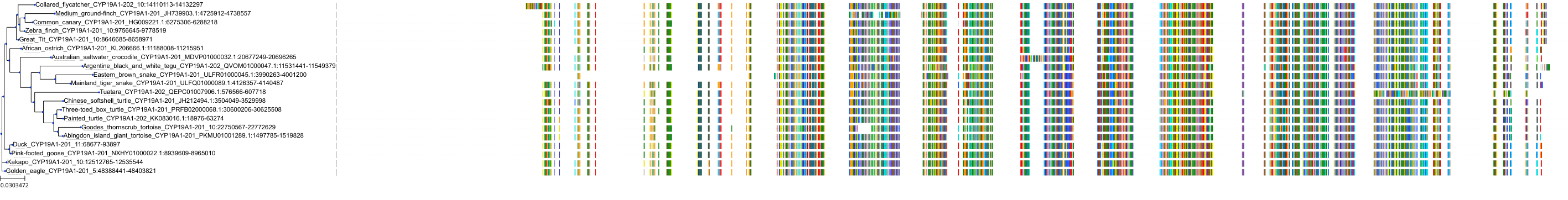
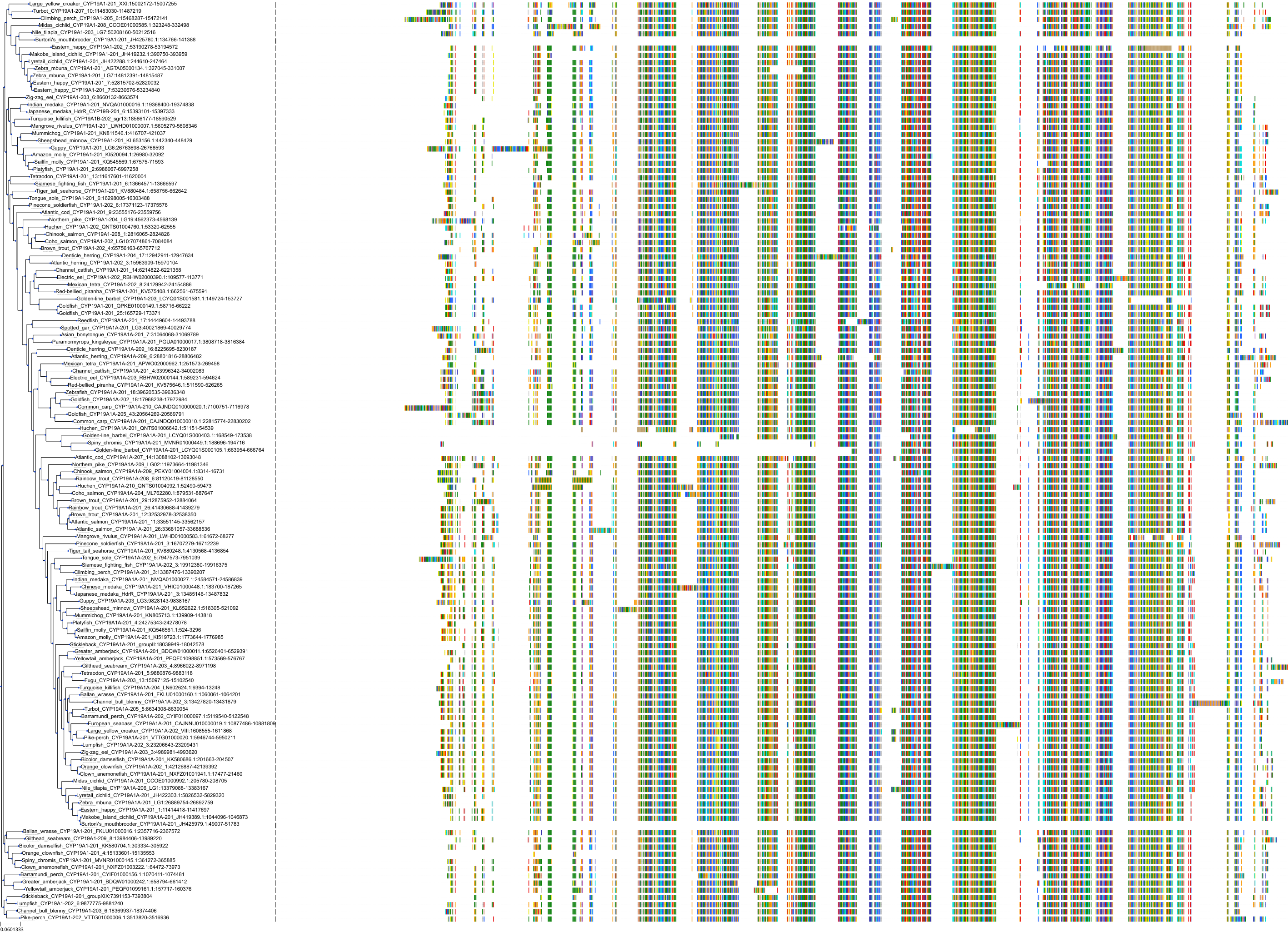
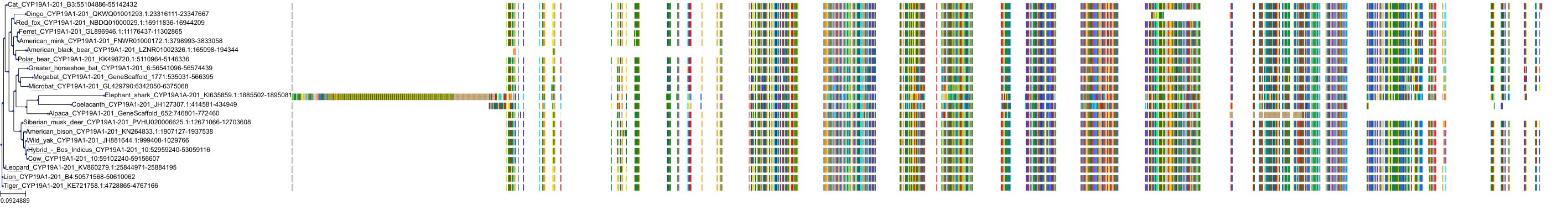
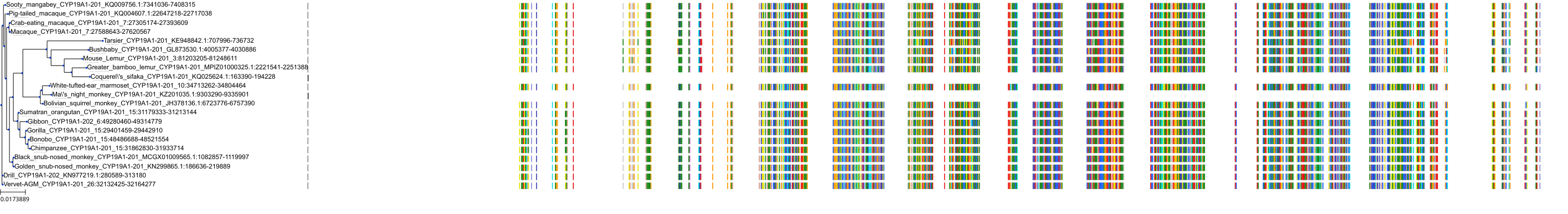
Target Conservation
|
Protein: Cytochrome P450 19A1 Description: Aromatase Organism : Homo sapiens P11511 ENSG00000137869 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 4953 |
| ChEMBL | CHEMBL1200374 |
| DrugBank | DB00990 |
| DrugCentral | 1122 |
| FDA SRS | NY22HMQ4BX |
| Human Metabolome Database | HMDB0015125 |
| Guide to Pharmacology | 7073 |
| PDB | EXM |
| PharmGKB | PA449563 |
| SureChEMBL | SCHEMBL6215 |
| ZINC | ZINC000003973334 |
 Homo sapiens
Homo sapiens