| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| ATC | A10XA01 |
| UNII | 0T93LG5NMK |
| EPA CompTox | DTXSID6048834 |
Structure
| InChI Key | LUBHDINQXIHVLS-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C16H14F3NO3S |
| Molecular Weight | 357.35 |
| AlogP | 3.56 |
| Hydrogen Bond Acceptor | 3.0 |
| Hydrogen Bond Donor | 1.0 |
| Number of Rotational Bond | 4.0 |
| Polar Surface Area | 49.77 |
| Molecular species | ACID |
| Aromatic Rings | 2.0 |
| Heavy Atoms | 24.0 |
Pharmacology
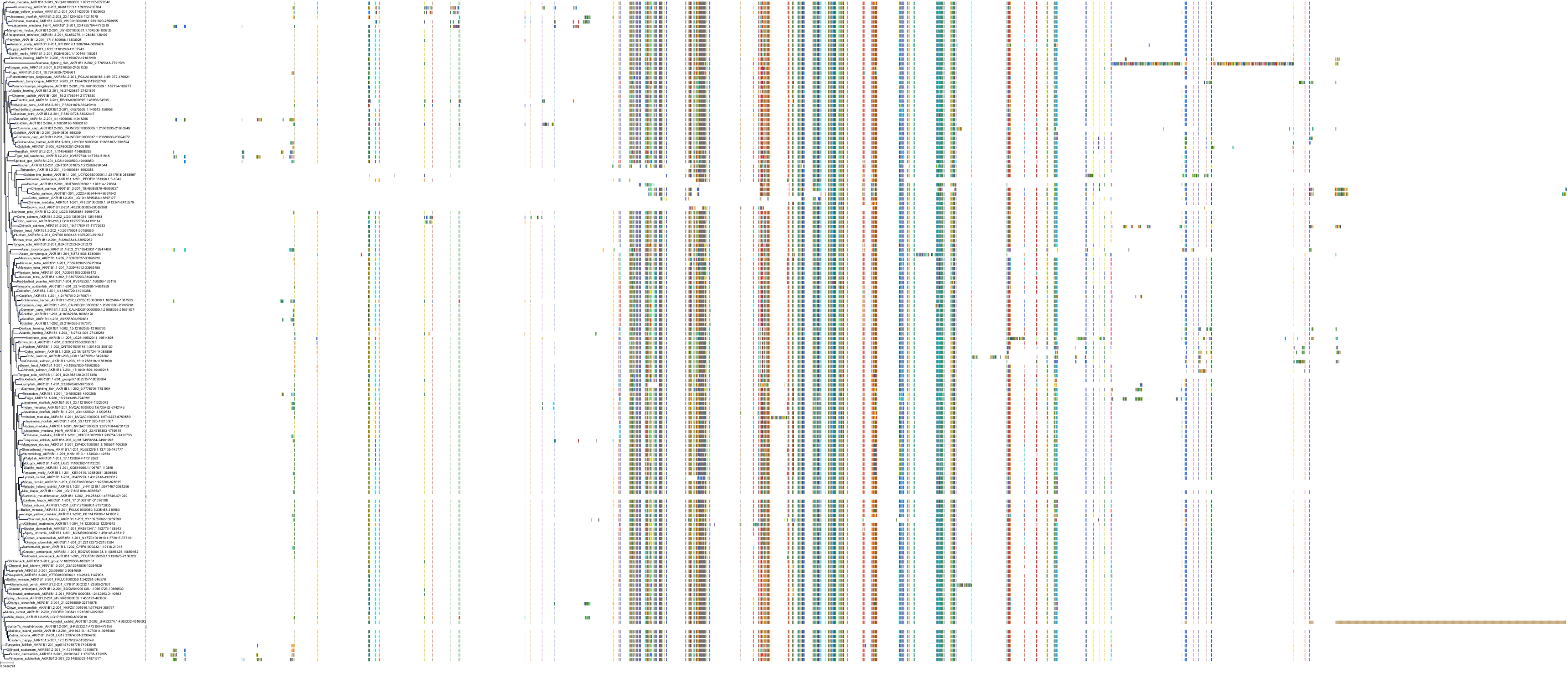
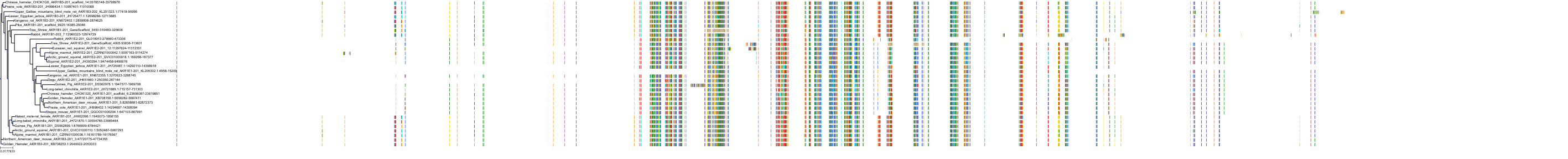
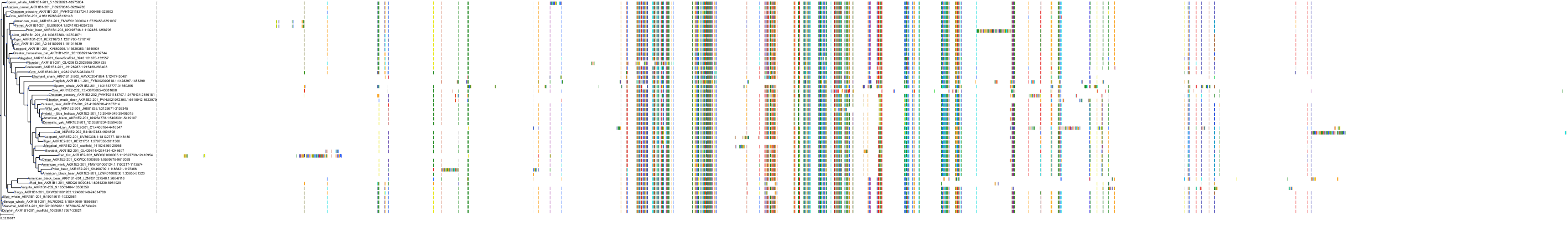
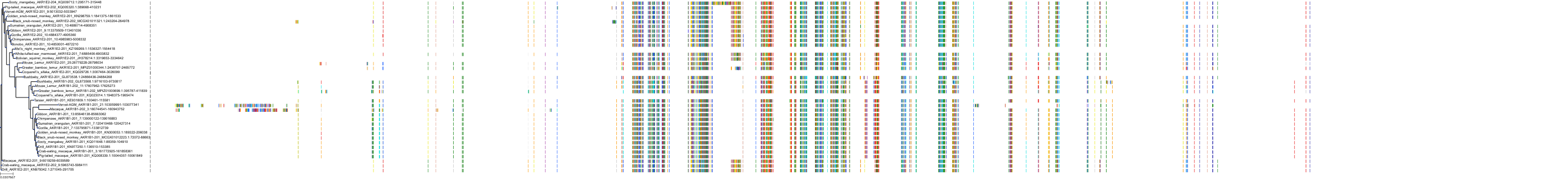
Target Conservation
|
Protein: Aldose reductase Description: Aldo-keto reductase family 1 member B1 Organism : Homo sapiens P15121 ENSG00000085662 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 48549 |
| ChEMBL | CHEMBL436 |
| DrugBank | DB02383 |
| DrugCentral | 2704 |
| FDA SRS | 0T93LG5NMK |
| Guide to Pharmacology | 7404 |
| KEGG | C01621 |
| PDB | TOL |
| PubChem | 53359 |
| SureChEMBL | SCHEMBL49326 |
| ZINC | ZINC000003780343 |
 Bos taurus
Bos taurus
 Canis lupus familiaris
Canis lupus familiaris
 Homo sapiens
Homo sapiens
 Ovis aries
Ovis aries
 Rattus norvegicus
Rattus norvegicus
 Sus scrofa
Sus scrofa