| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| UNII | C5INA23HXF |
| EPA CompTox | DTXSID7040152 |
Structure
| InChI Key | BJRNKVDFDLYUGJ-RMPHRYRLSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C12H16O7 |
| Molecular Weight | 272.25 |
| AlogP | -1.43 |
| Hydrogen Bond Acceptor | 7.0 |
| Hydrogen Bond Donor | 5.0 |
| Number of Rotational Bond | 3.0 |
| Polar Surface Area | 119.61 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 1.0 |
| Heavy Atoms | 19.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Tyrosinase inhibitor | INHIBITOR | PubMed |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Enzyme
Hydrolase
|
- | - | - | - | 6.63-9.16 | |
|
Enzyme
Oxidoreductase
|
- | - | - | - | -1.5-146.5 | |
|
Transporter
Electrochemical transporter
SLC superfamily of solute carriers
SLC21/SLCO family of organic anion transporting polypeptides
|
- | - | - | - | 103.74-113.42 |
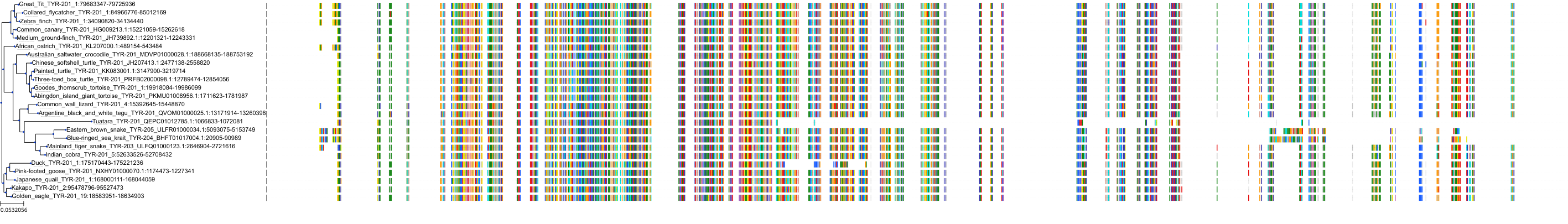
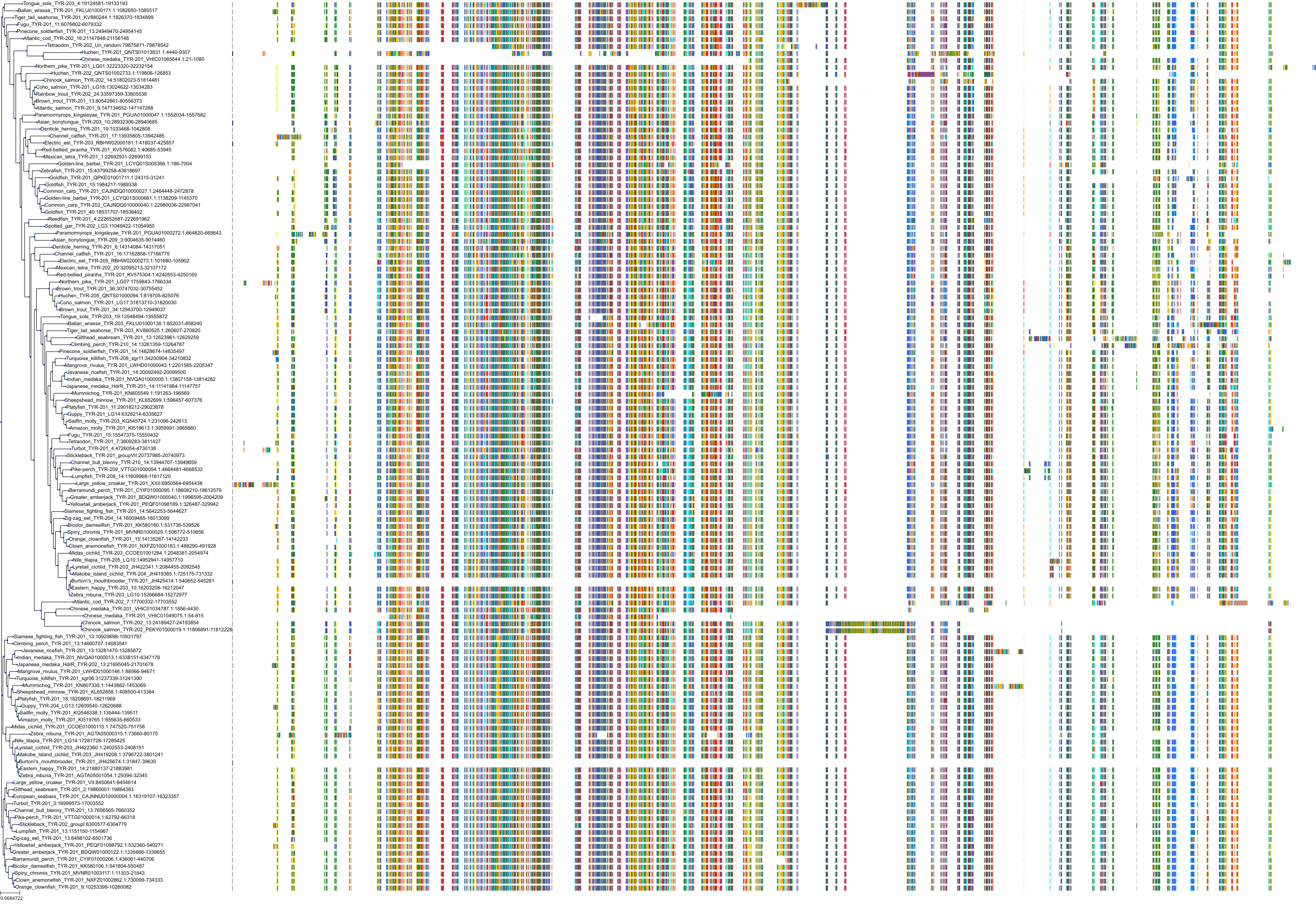
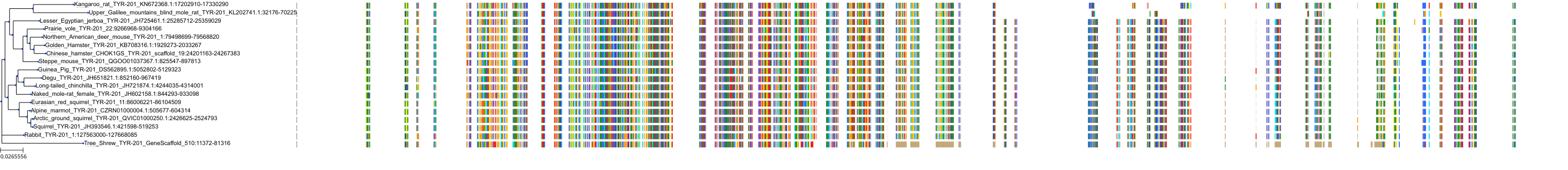
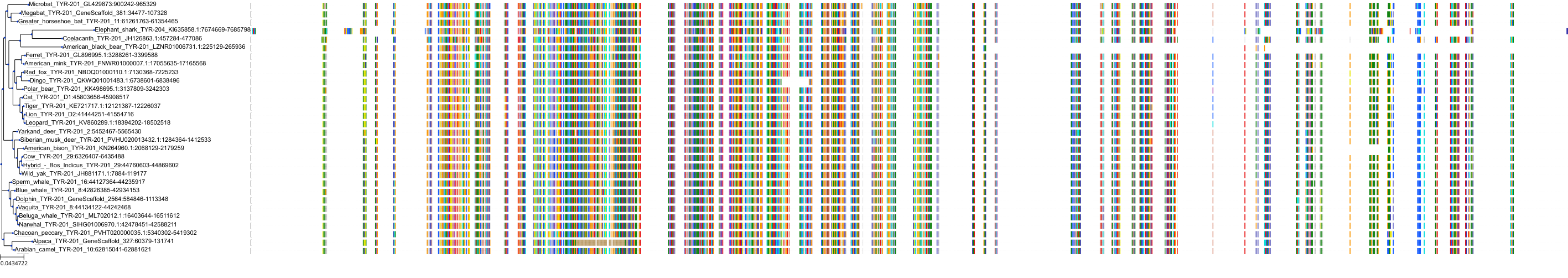
Target Conservation
|
Protein: Tyrosinase Description: Tyrosinase Organism : Homo sapiens P14679 ENSG00000077498 |
||||
Related Entries
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 18305 |
| ChEMBL | CHEMBL232202 |
| DrugBank | DB11217 |
| DrugCentral | 4267 |
| FDA SRS | C5INA23HXF |
| KEGG | C06186 |
| PubChem | 440936 |
| SureChEMBL | SCHEMBL36351 |
| ZINC | ZINC000000518554 |
 Agaricus bisporus
Agaricus bisporus
 Cricetulus griseus
Cricetulus griseus
 Electrophorus electricus
Electrophorus electricus
 Equus caballus
Equus caballus
 Homo sapiens
Homo sapiens
 Mus musculus
Mus musculus