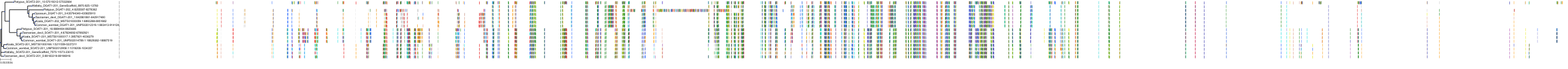Structure
| InChI Key | PKKNCEXEVUFFFI-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C27H39N3O |
| Molecular Weight | 421.63 |
| AlogP | 6.63 |
| Hydrogen Bond Acceptor | 2.0 |
| Hydrogen Bond Donor | 2.0 |
| Number of Rotational Bond | 7.0 |
| Polar Surface Area | 44.37 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 2.0 |
| Heavy Atoms | 31.0 |
Pharmacology
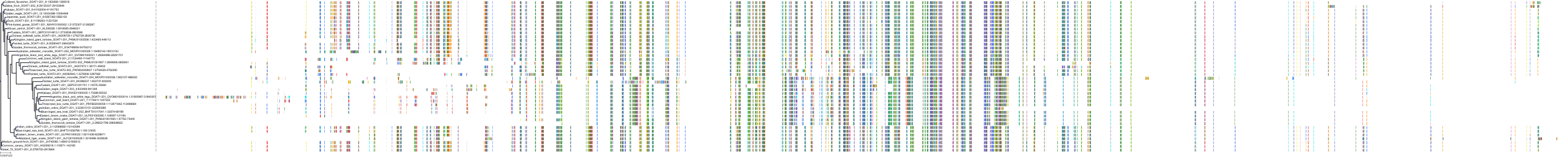
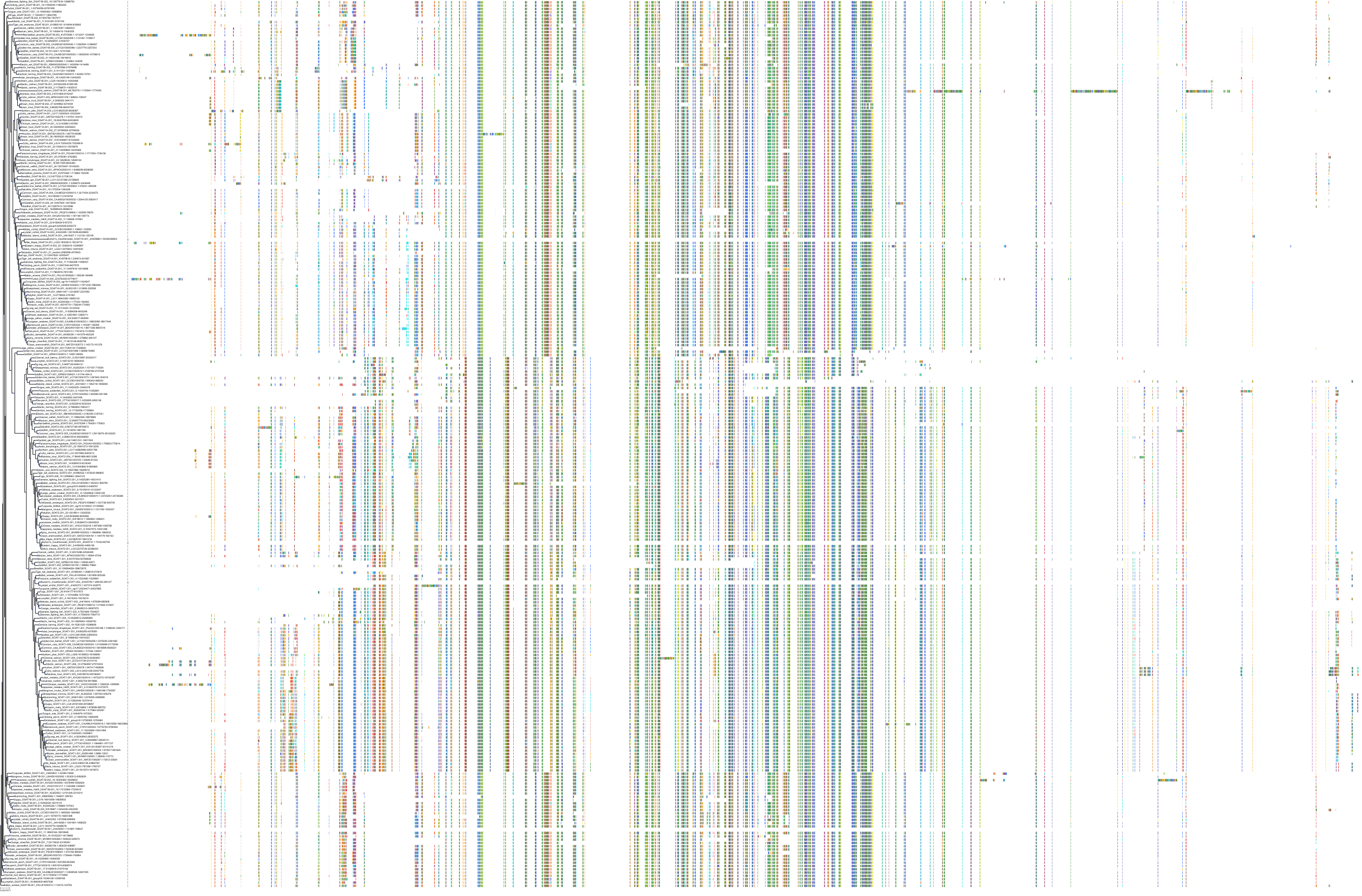
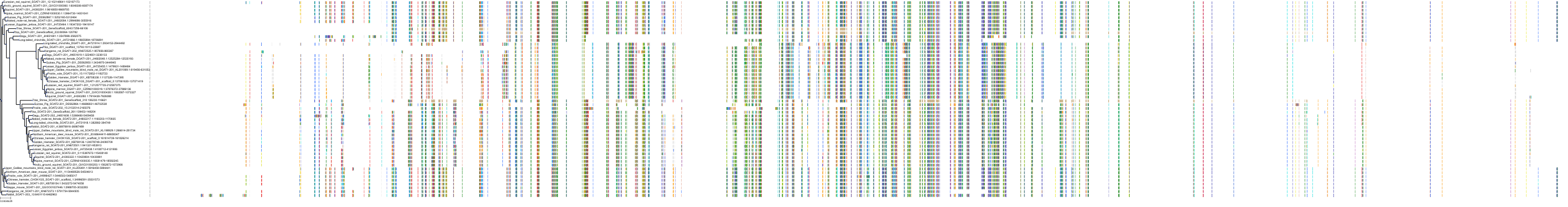
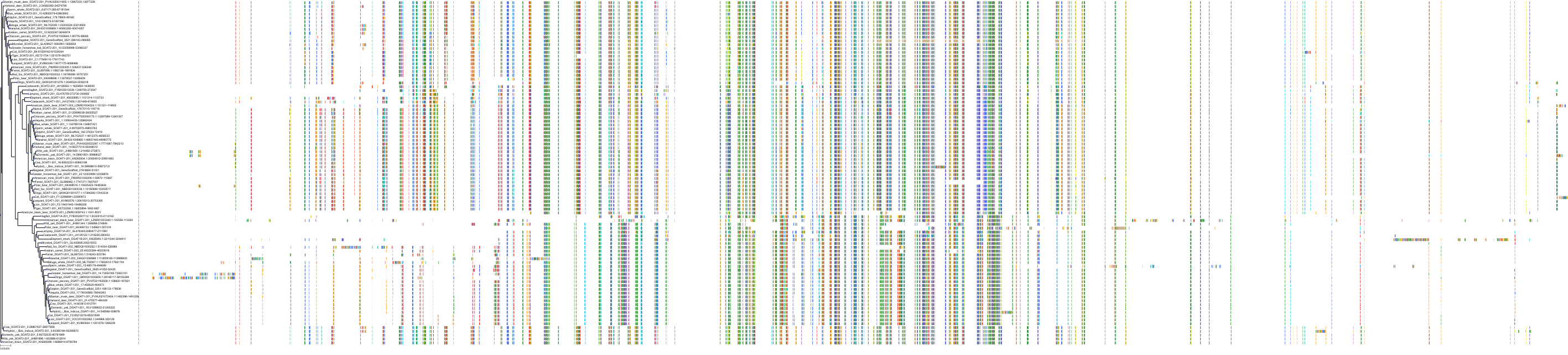
Target Conservation
|
Protein: Acyl coenzyme A:cholesterol acyltransferase 1 Description: Sterol O-acyltransferase 1 Organism : Homo sapiens P35610 ENSG00000057252 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL46423 |
| DrugBank | DB16254 |
| FDA SRS | VK9OS8R205 |
| PDB | ROV |
| PubChem | 131679 |
| SureChEMBL | SCHEMBL1119314 |
 Oryctolagus cuniculus
Oryctolagus cuniculus