| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| UNII | ANC71R916O |
| EPA CompTox | DTXSID20153789 |
Structure
| InChI Key | SQSZANZGUXWJEA-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C23H25ClFN7O |
| Molecular Weight | 469.95 |
| AlogP | 4.03 |
| Hydrogen Bond Acceptor | 7.0 |
| Hydrogen Bond Donor | 2.0 |
| Number of Rotational Bond | 6.0 |
| Polar Surface Area | 83.37 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 4.0 |
| Heavy Atoms | 33.0 |
Pharmacology
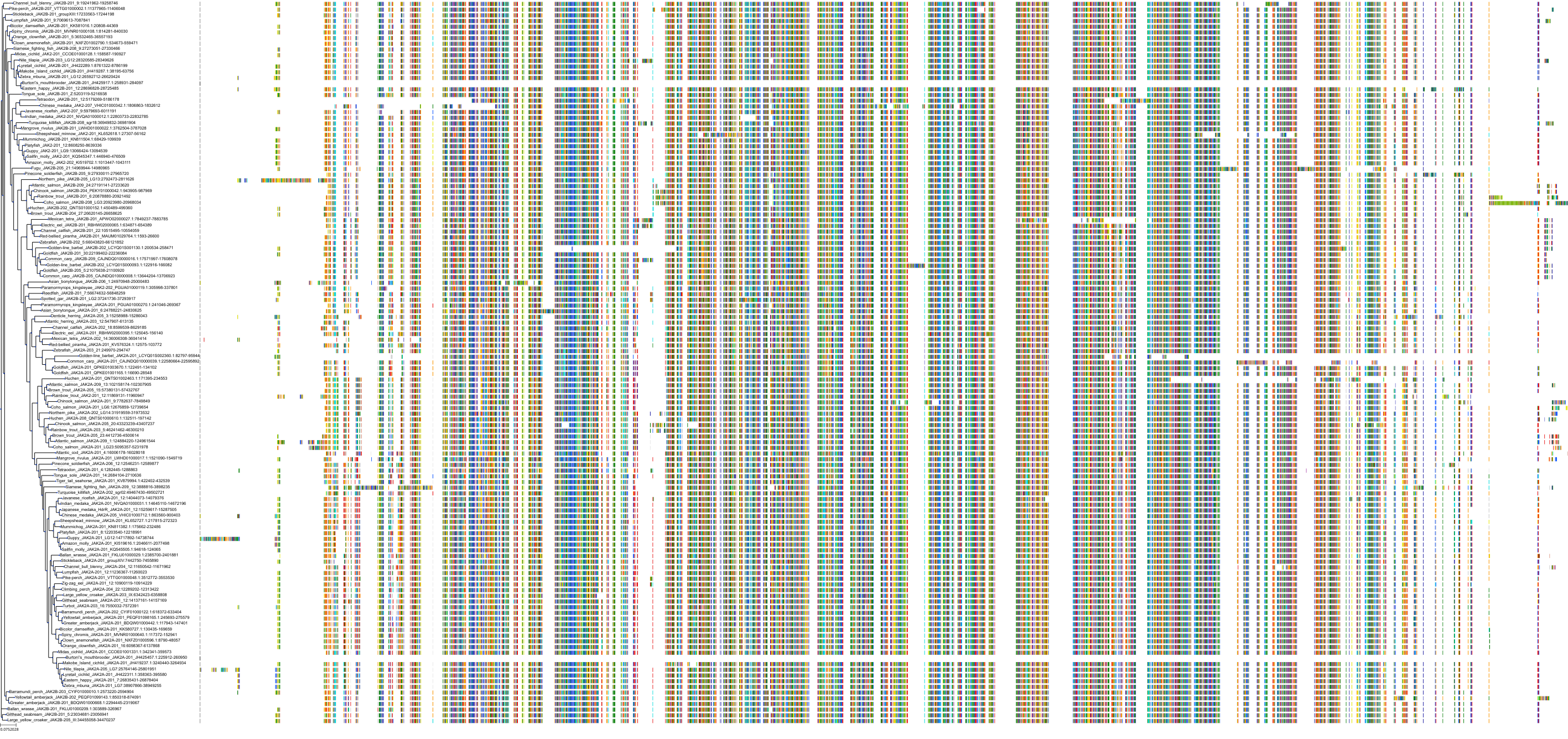
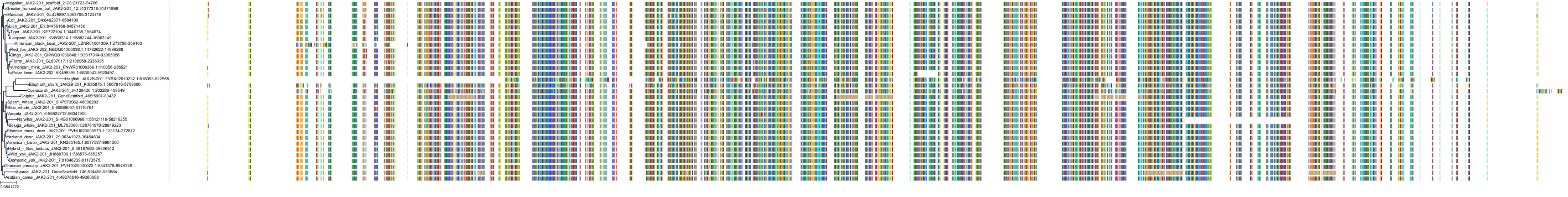
Target Conservation
|
Protein: Tyrosine-protein kinase JAK2 Description: Tyrosine-protein kinase JAK2 Organism : Homo sapiens O60674 ENSG00000096968 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL2107823 |
| DrugBank | DB13040 |
| FDA SRS | ANC71R916O |
| Guide to Pharmacology | 7909 |
| PubChem | 46213929 |
| SureChEMBL | SCHEMBL2513132 |
| ZINC | ZINC000068245097 |