| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| UNII | UA8SE1325T |
| EPA CompTox | DTXSID8046799 |
Structure
| InChI Key | ZPLQIPFOCGIIHV-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C5H4ClNO2 |
| Molecular Weight | 145.55 |
| AlogP | 1.15 |
| Hydrogen Bond Acceptor | 3.0 |
| Hydrogen Bond Donor | 2.0 |
| Polar Surface Area | 53.35 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 1.0 |
| Heavy Atoms | 9.0 |
Pharmacology
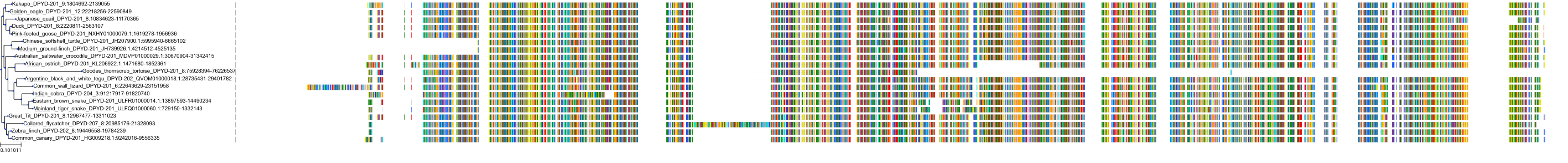
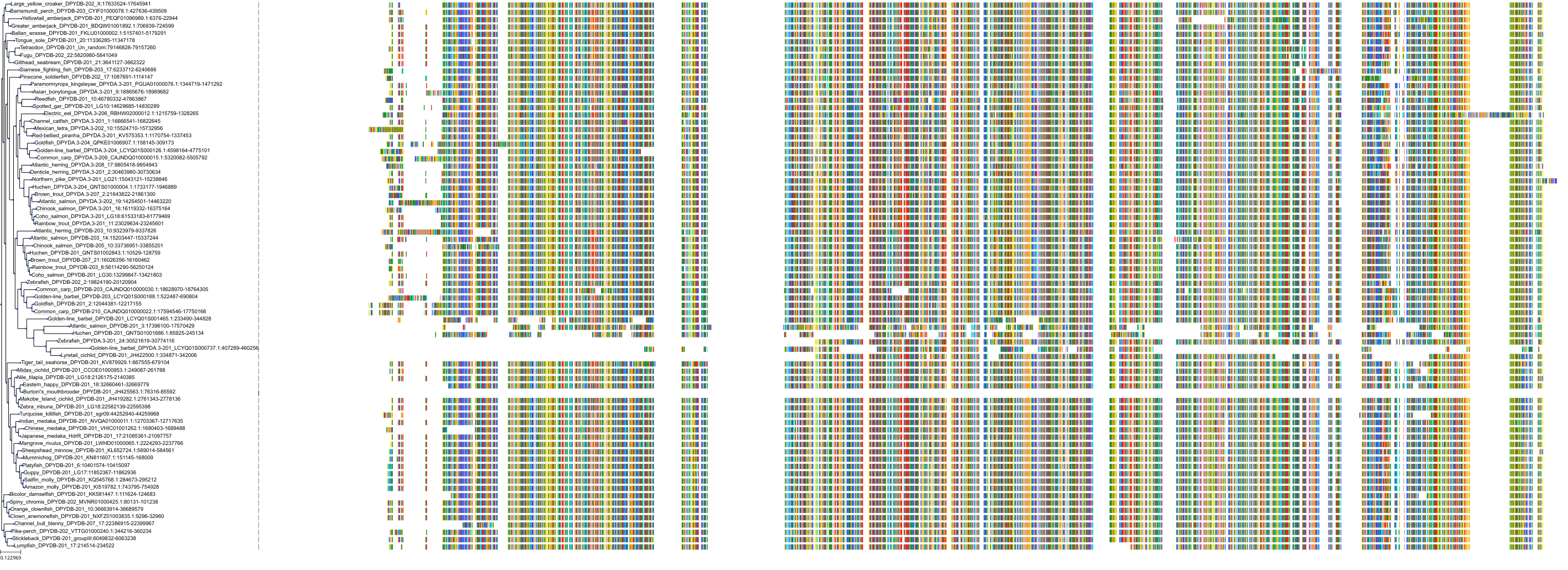
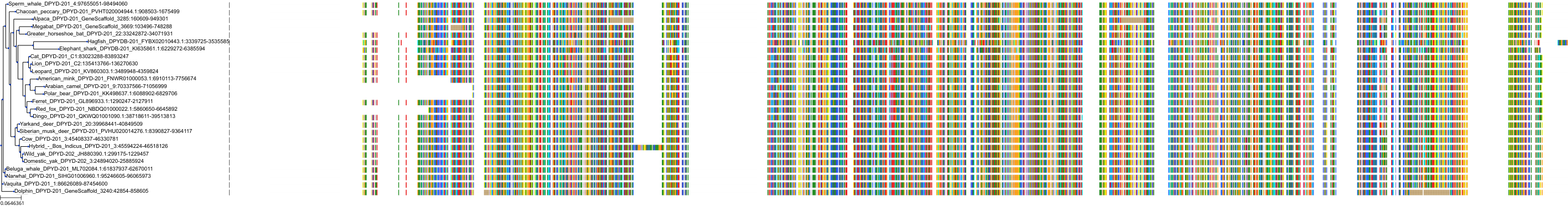
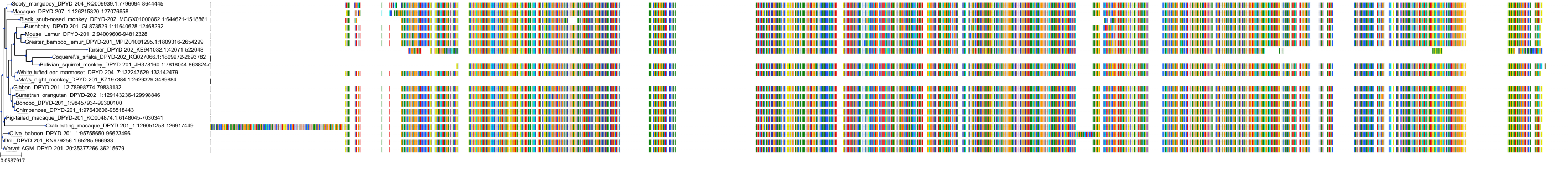
Target Conservation
|
Protein: Dihydropyrimidine dehydrogenase Description: Dihydropyrimidine dehydrogenase [NADP(+)] Organism : Homo sapiens Q12882 ENSG00000188641 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEBI | 31652 |
| ChEMBL | CHEMBL1730601 |
| DrugBank | DB09257 |
| DrugCentral | 1293 |
| FDA SRS | UA8SE1325T |
| PubChem | 54679224 |
| SureChEMBL | SCHEMBL124438 |
| ZINC | ZINC000013831809 |