Structure
| InChI Key | QINPEPAQOBZPOF-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C25H25ClN6O4S |
| Molecular Weight | 541.03 |
| AlogP | 4.51 |
| Hydrogen Bond Acceptor | 8.0 |
| Hydrogen Bond Donor | 4.0 |
| Number of Rotational Bond | 8.0 |
| Polar Surface Area | 148.33 |
| Molecular species | ACID |
| Aromatic Rings | 4.0 |
| Heavy Atoms | 37.0 |
Pharmacology
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Enzyme
Transferase
|
- | 23-383 | - | - | - |
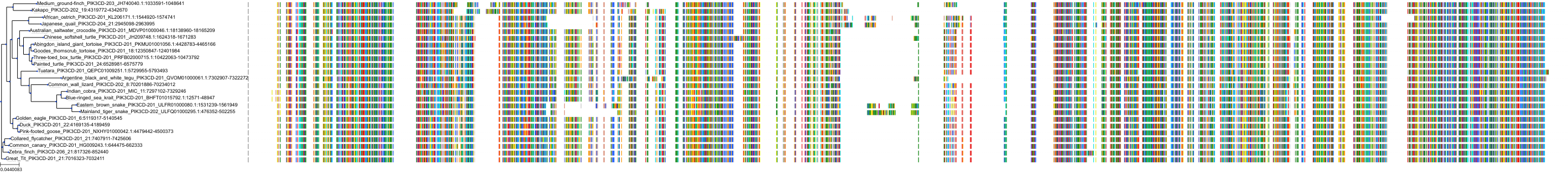
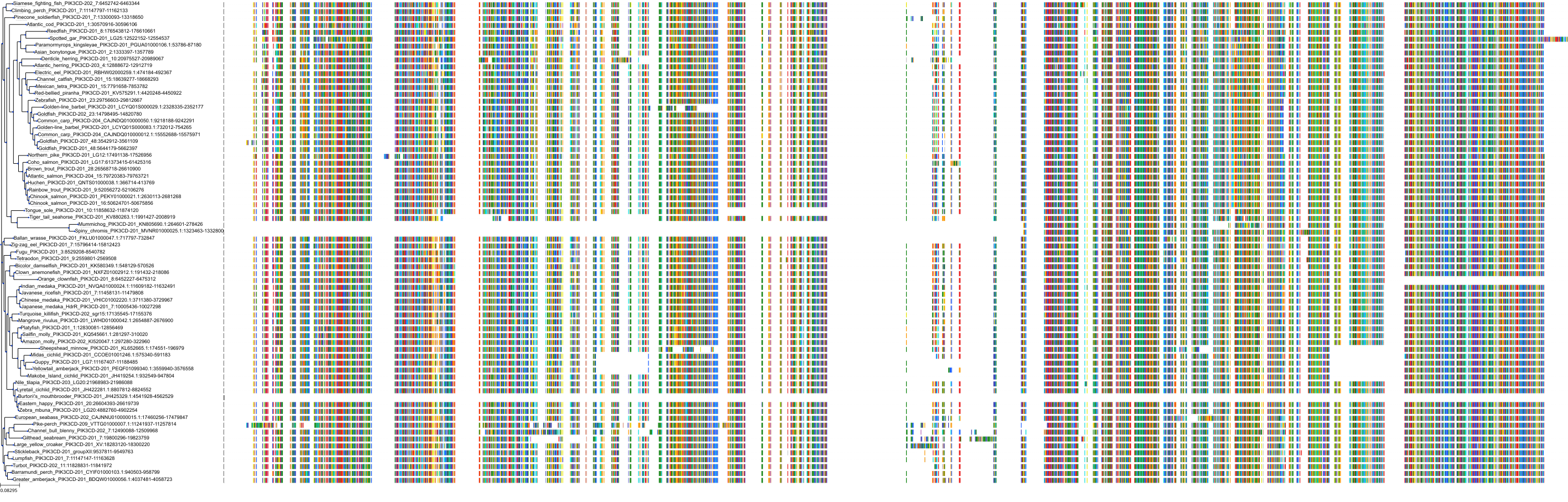
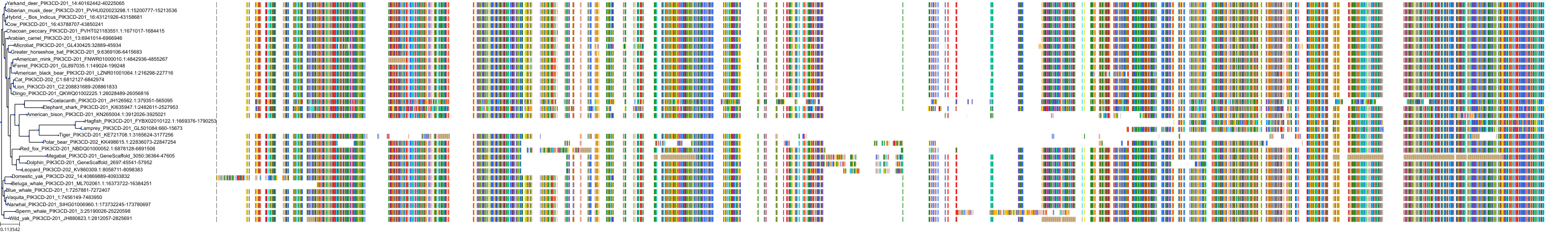
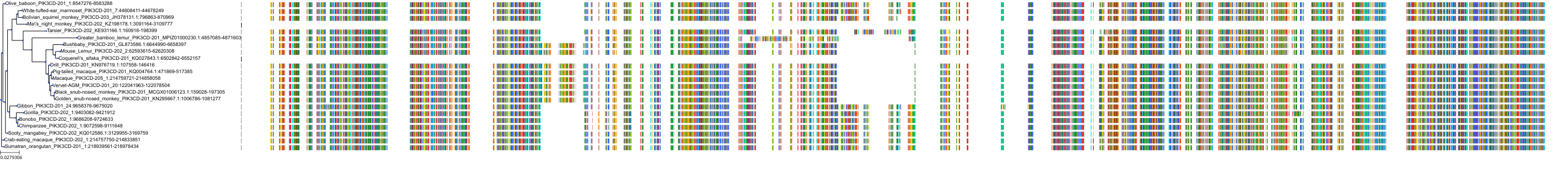
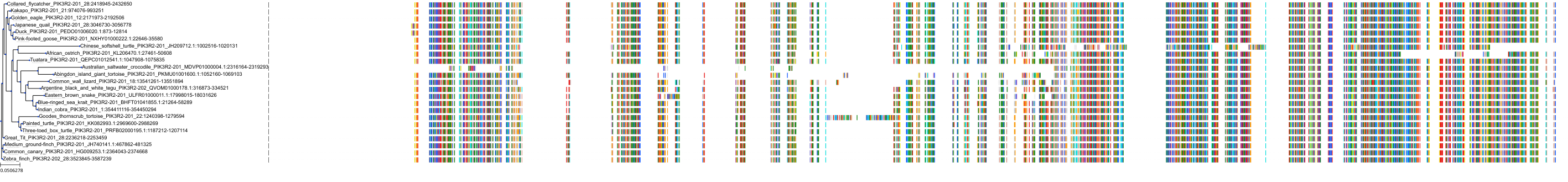
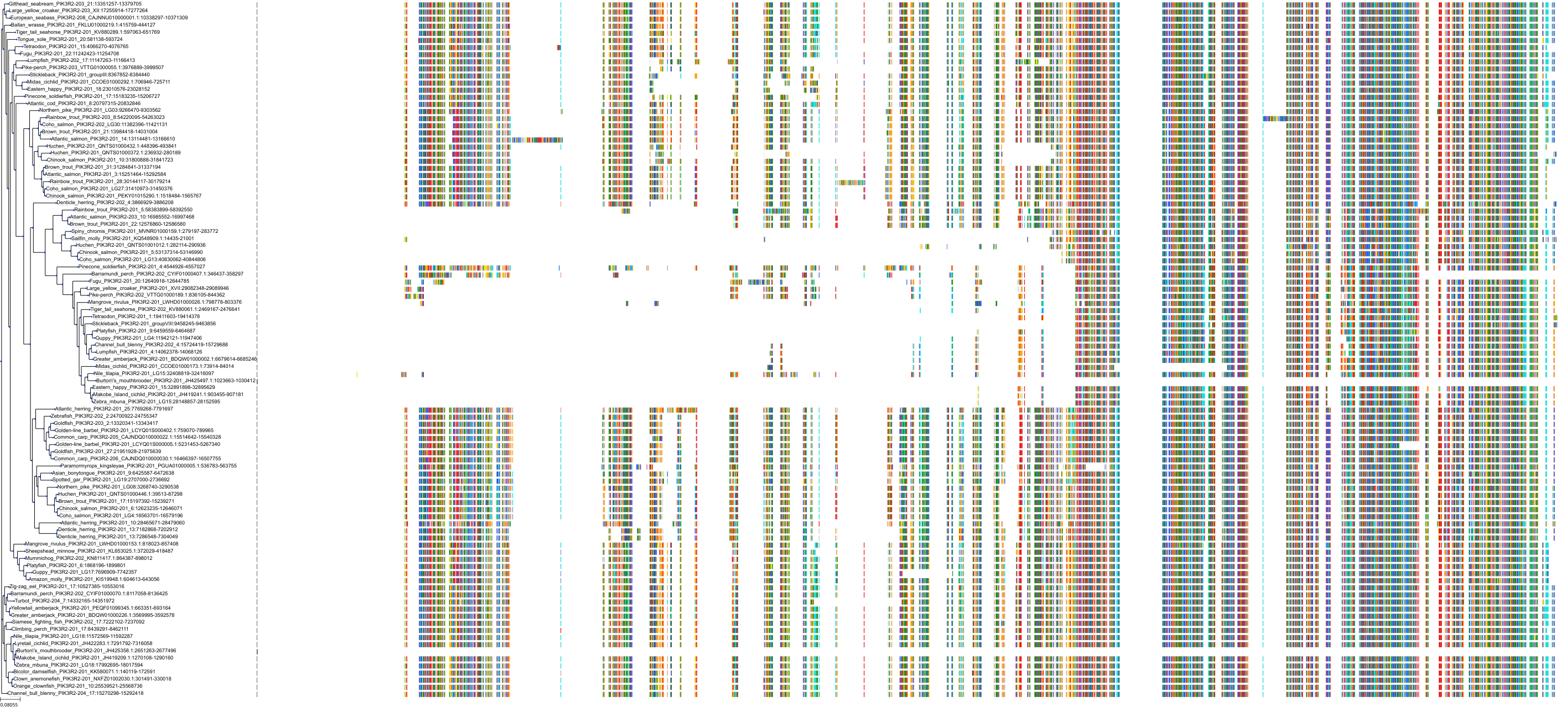
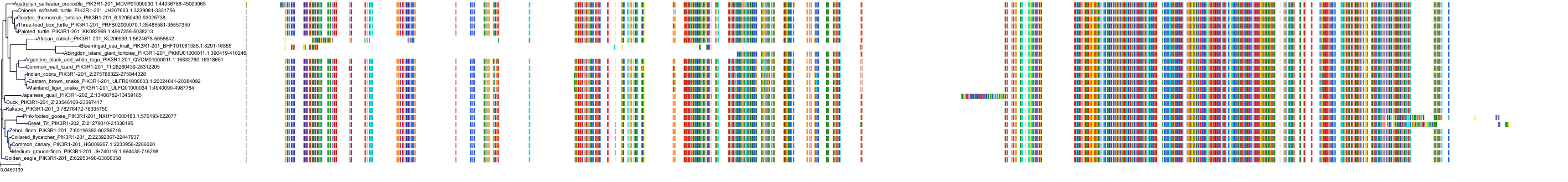
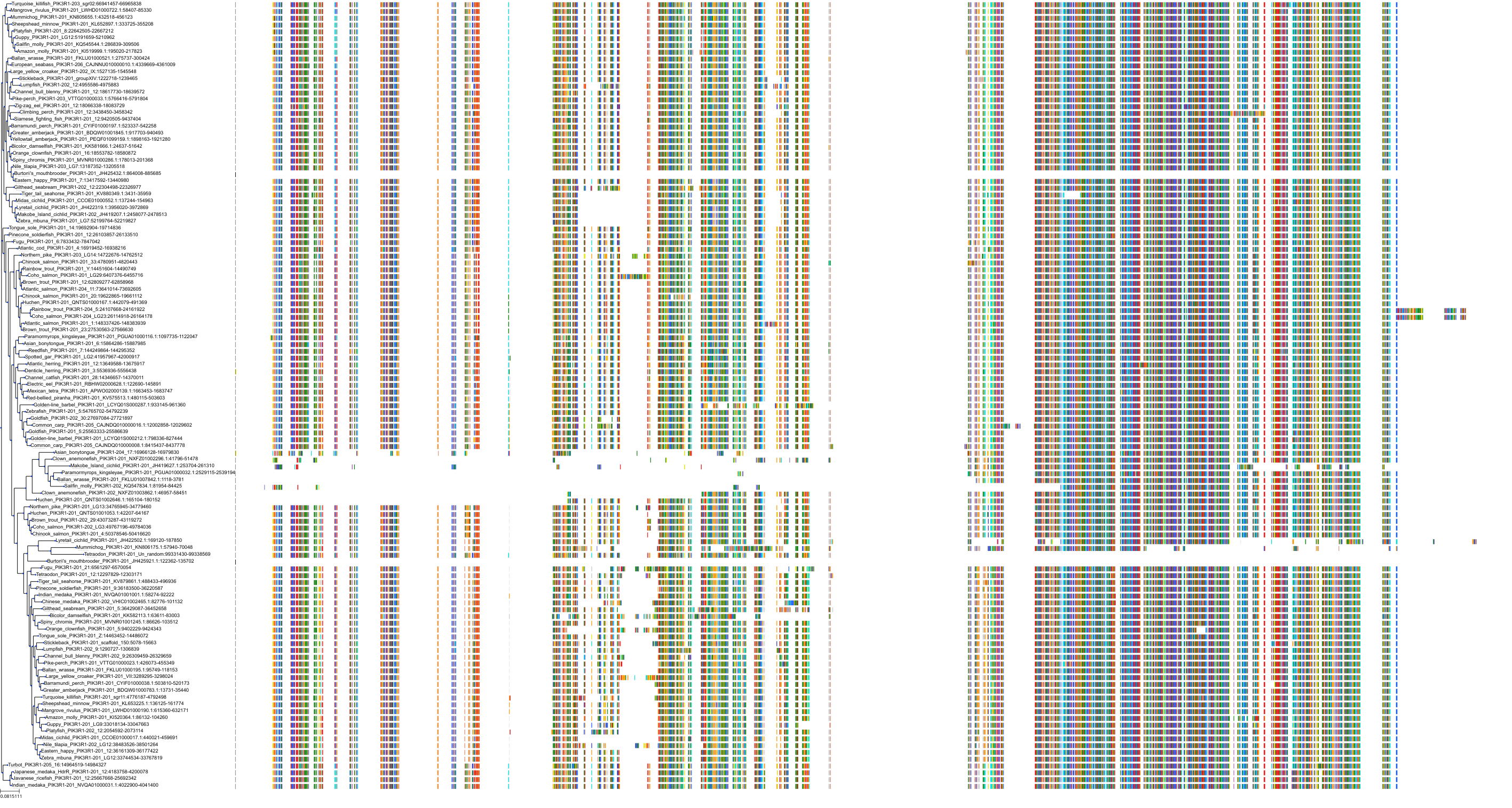
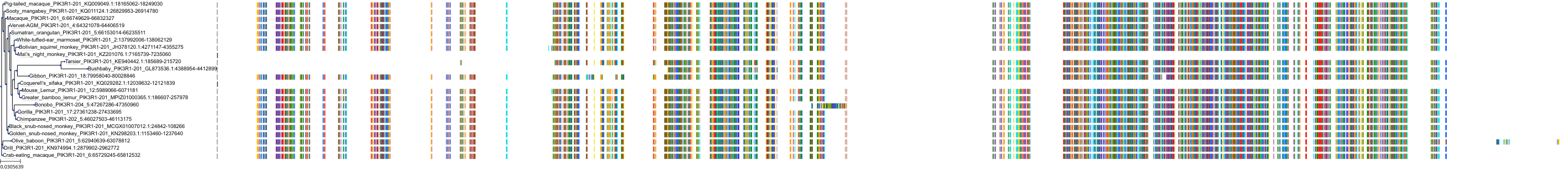
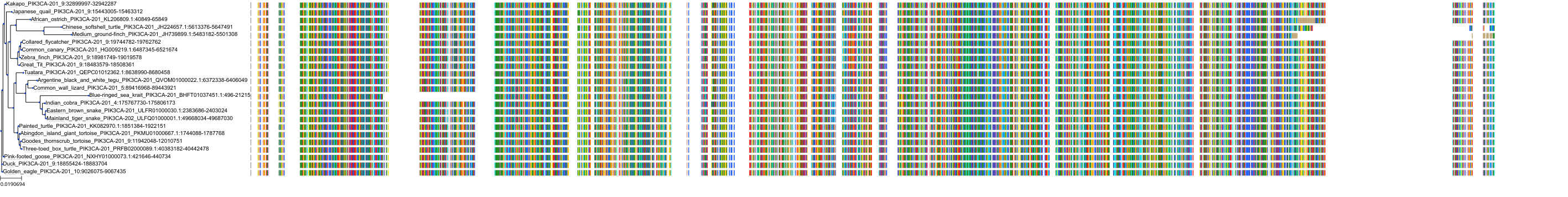
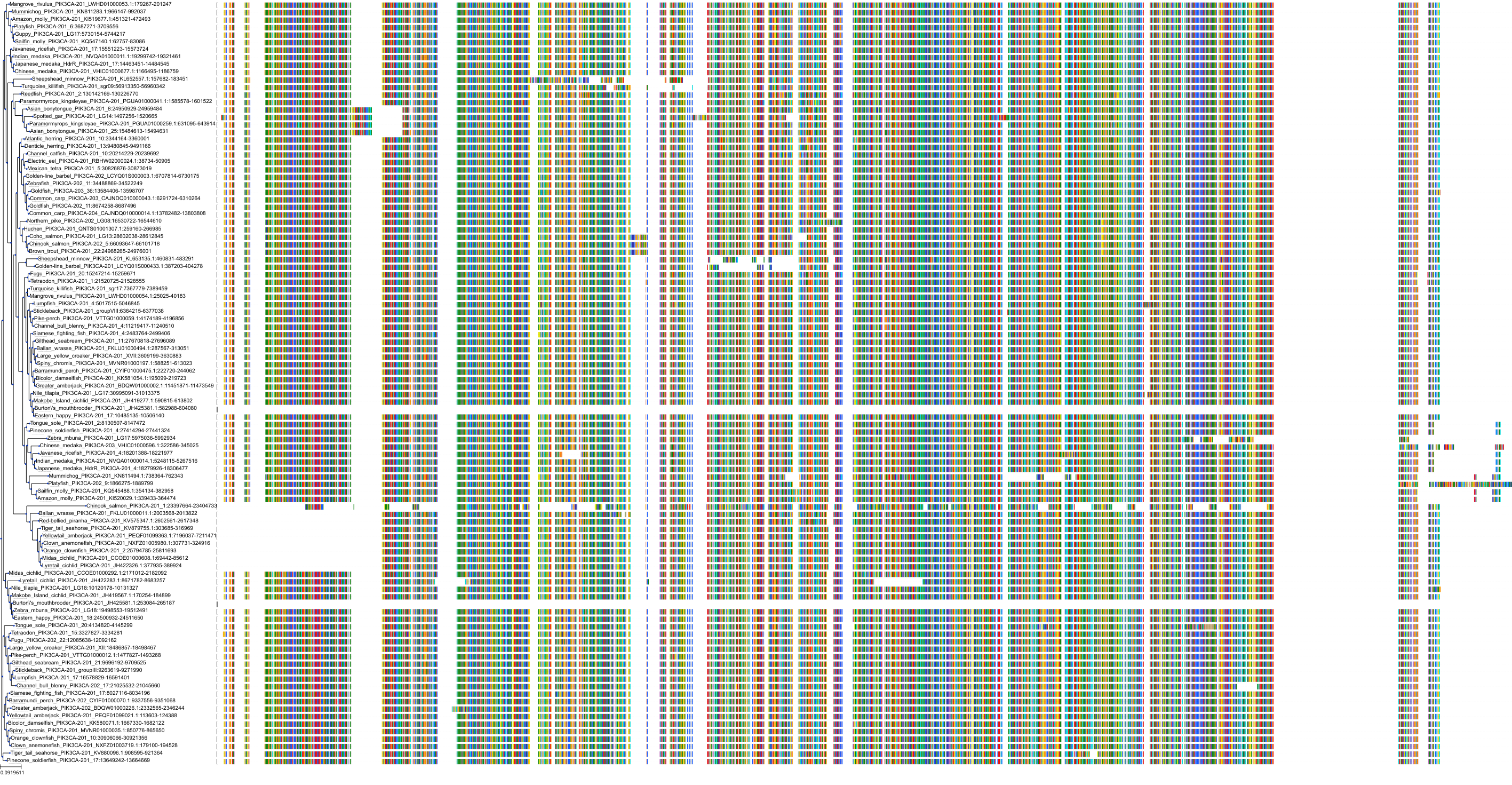
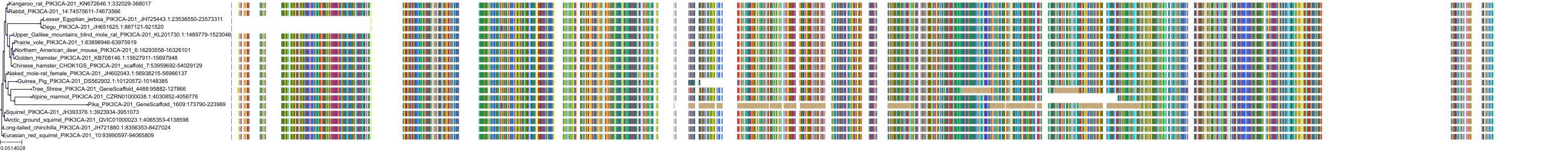
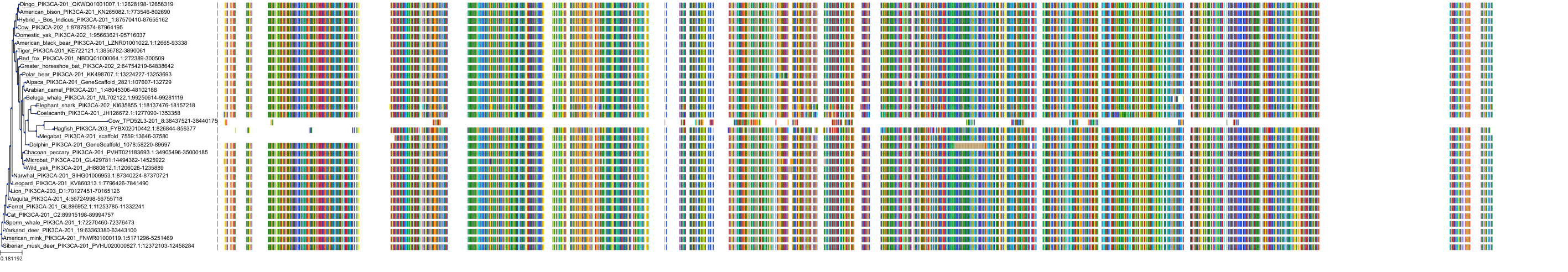
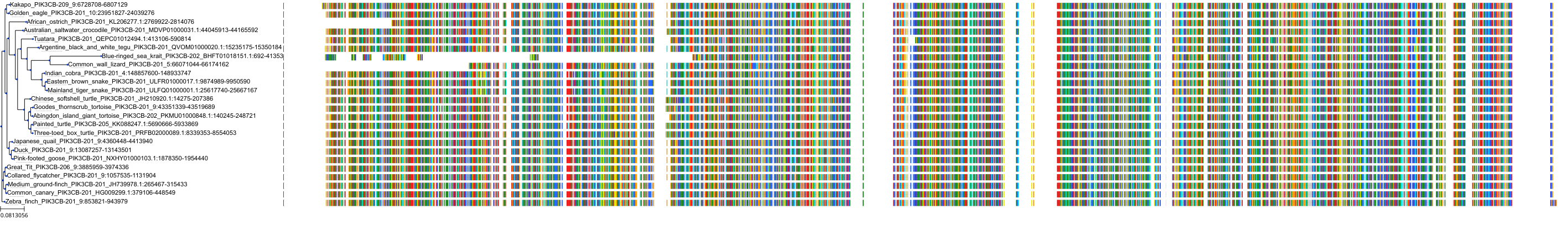
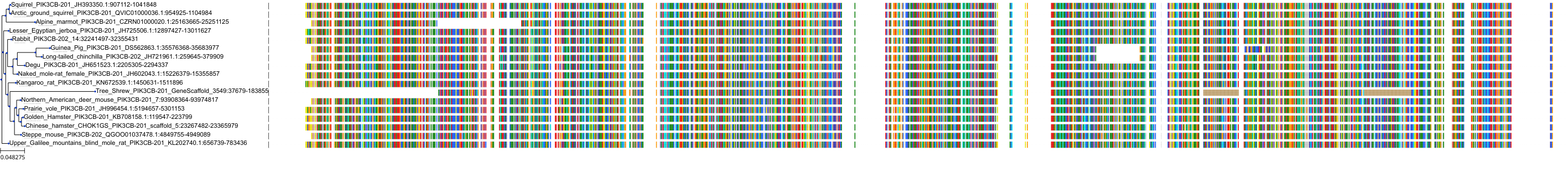
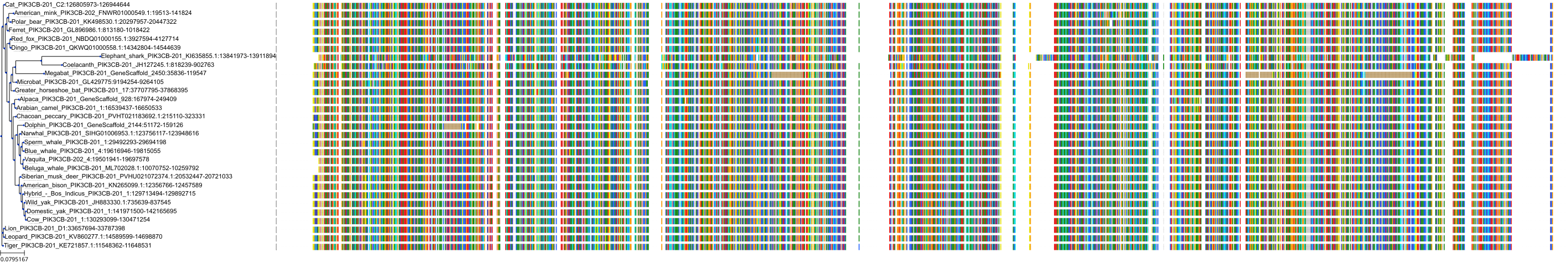
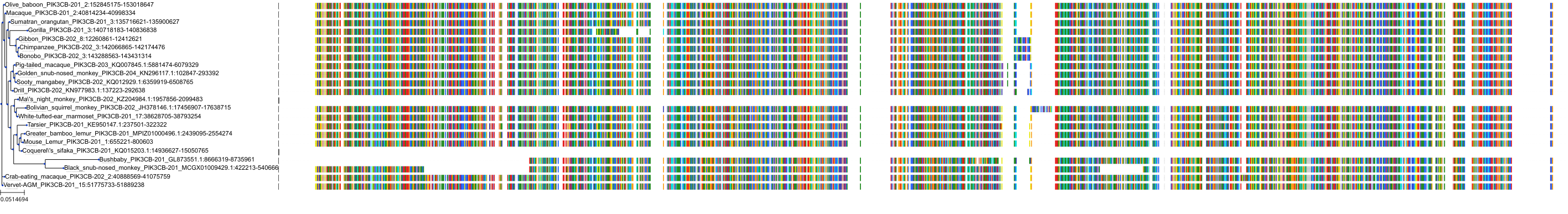
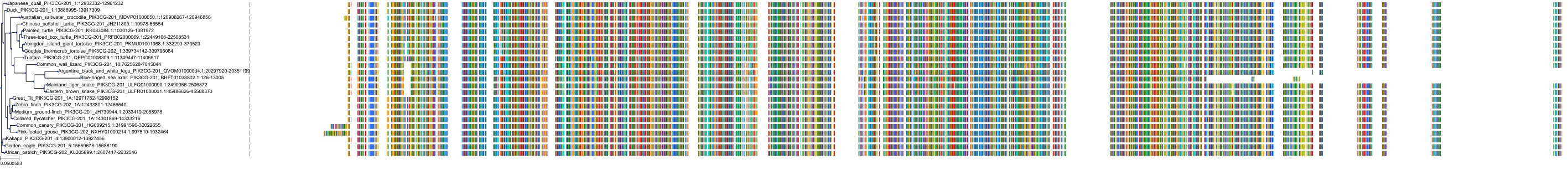
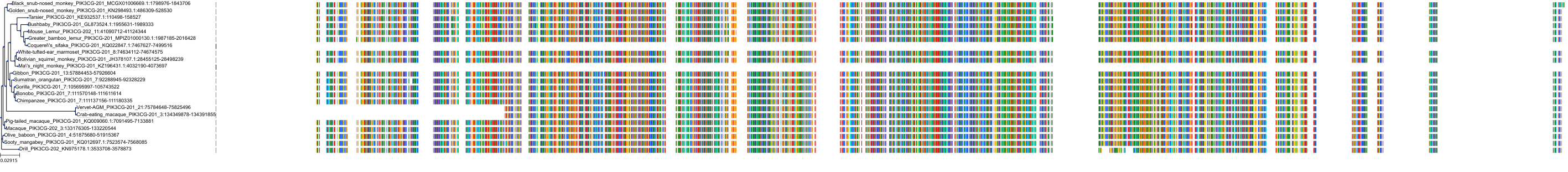
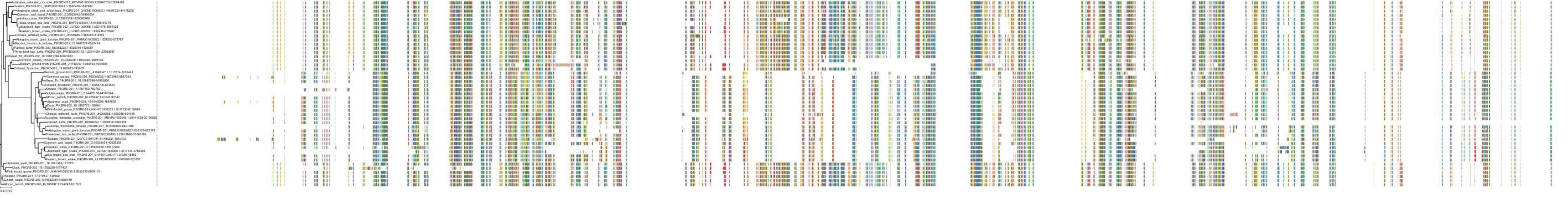
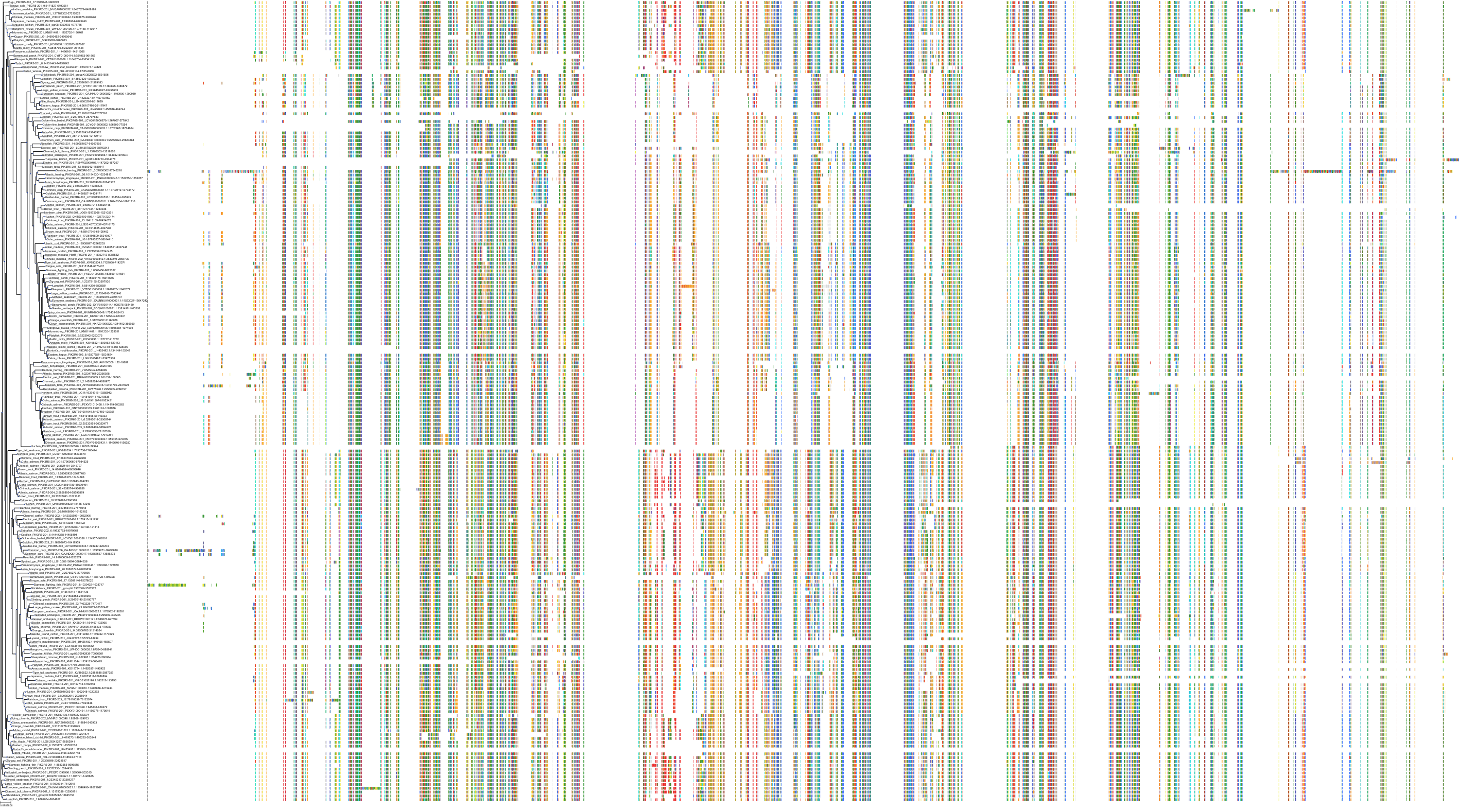
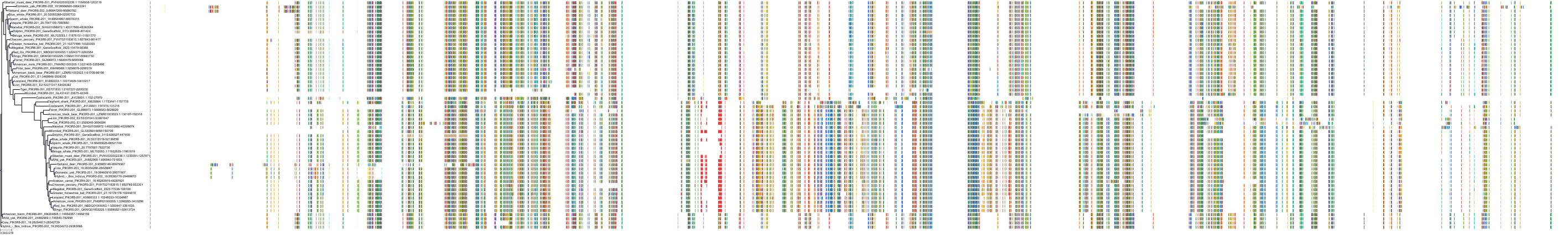
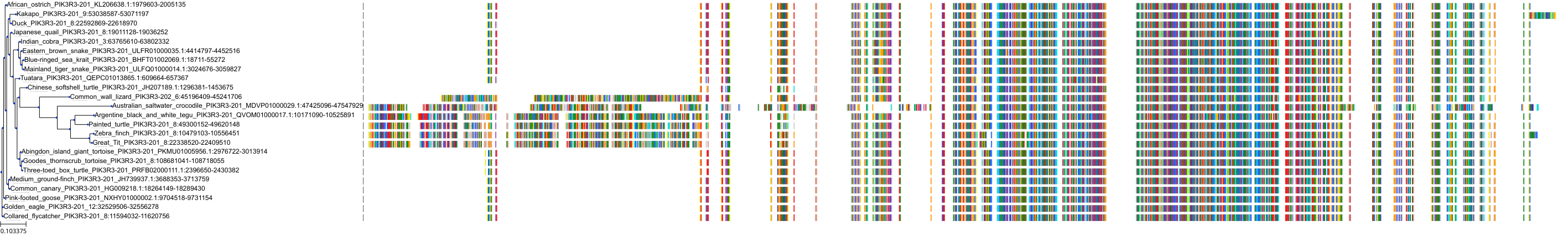
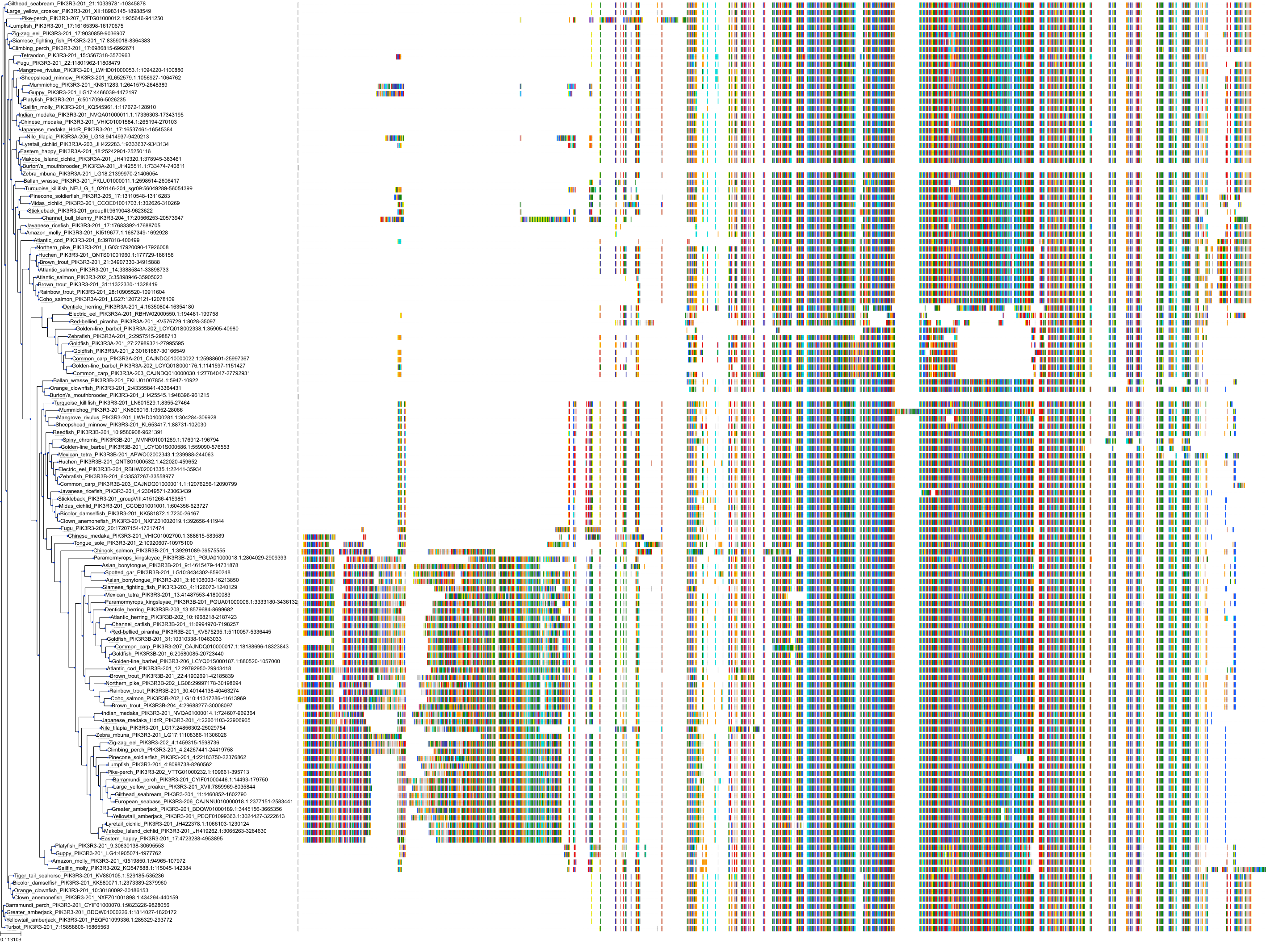
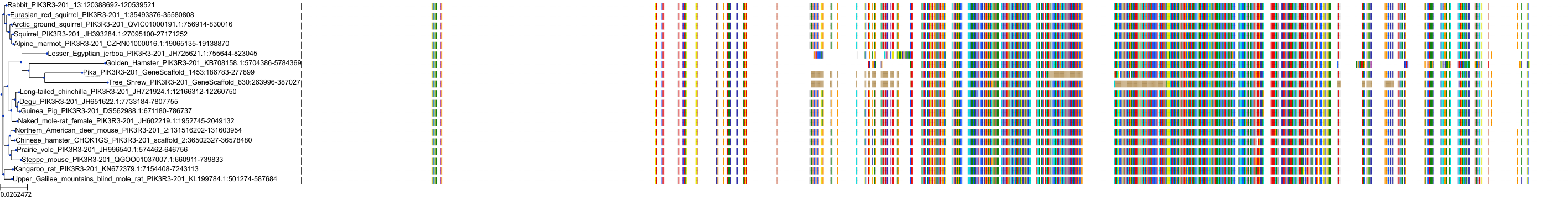
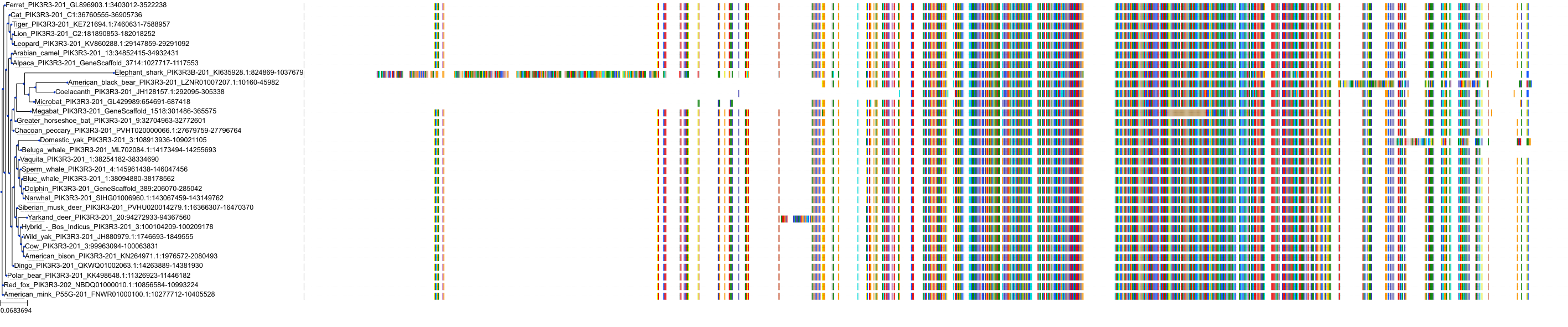
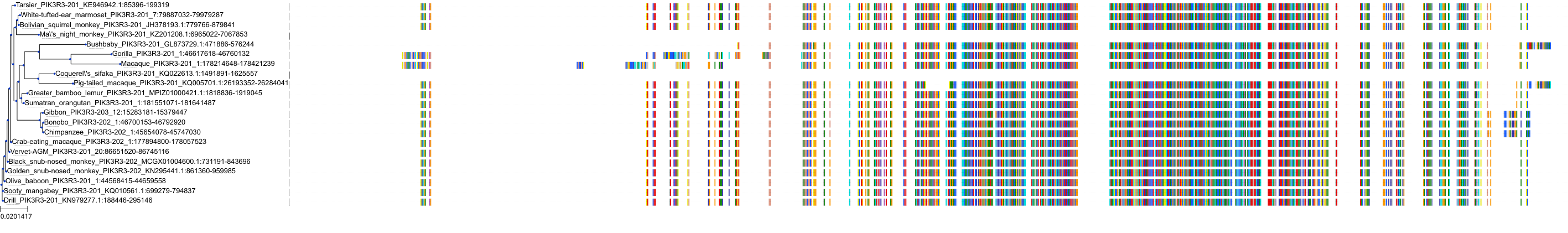
Target Conservation
|
Protein: PI3-kinase class I Description: Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform Organism : Homo sapiens O00329 ENSG00000171608 |
||||
|
Protein: PI3-kinase class I Description: Phosphatidylinositol 3-kinase regulatory subunit beta Organism : Homo sapiens O00459 ENSG00000105647 |
||||
|
Protein: PI3-kinase class I Description: Phosphatidylinositol 3-kinase regulatory subunit alpha Organism : Homo sapiens P27986 ENSG00000145675 |
||||
|
Protein: PI3-kinase class I Description: Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform Organism : Homo sapiens P42336 ENSG00000121879 |
||||
|
Protein: PI3-kinase class I Description: Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform Organism : Homo sapiens P42338 ENSG00000051382 |
||||
|
Protein: PI3-kinase class I Description: Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform Organism : Homo sapiens P48736 ENSG00000105851 |
||||
|
Protein: PI3-kinase class I Description: Phosphoinositide 3-kinase regulatory subunit 5 Organism : Homo sapiens Q8WYR1 ENSG00000141506 |
||||
|
Protein: PI3-kinase class I Description: Phosphatidylinositol 3-kinase regulatory subunit gamma Organism : Homo sapiens Q92569 ENSG00000117461 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL3360203 |
| DrugBank | DB11772 |
| FDA SRS | 60ES45KTMK |
| PubChem | 56599306 |
| SureChEMBL | SCHEMBL189422 |
| ZINC | ZINC000100472223 |
 Homo sapiens
Homo sapiens