Structure
| InChI Key | MMNNTJYFHUDSKL-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C24H19ClN4O4 |
| Molecular Weight | 462.89 |
| AlogP | 4.56 |
| Hydrogen Bond Acceptor | 5.0 |
| Hydrogen Bond Donor | 3.0 |
| Number of Rotational Bond | 3.0 |
| Polar Surface Area | 107.55 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 4.0 |
| Heavy Atoms | 33.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Serine/threonine-protein kinase B-raf inhibitor | INHIBITOR | PubMed Other Other PubMed |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Enzyme
Kinase
Protein Kinase
TKL protein kinase group
TKL protein kinase RAF family
|
- | 6 | - | - | - |
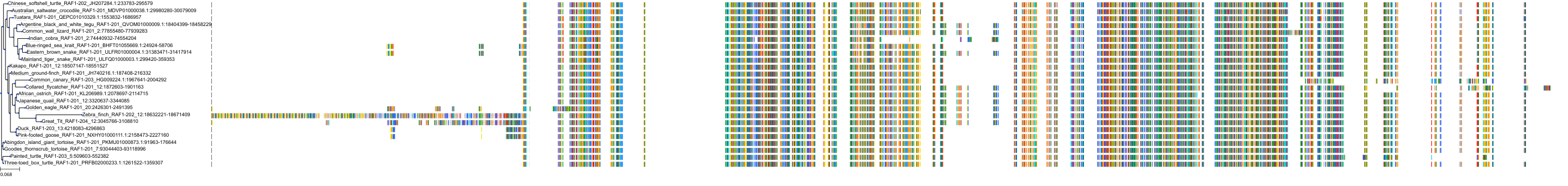
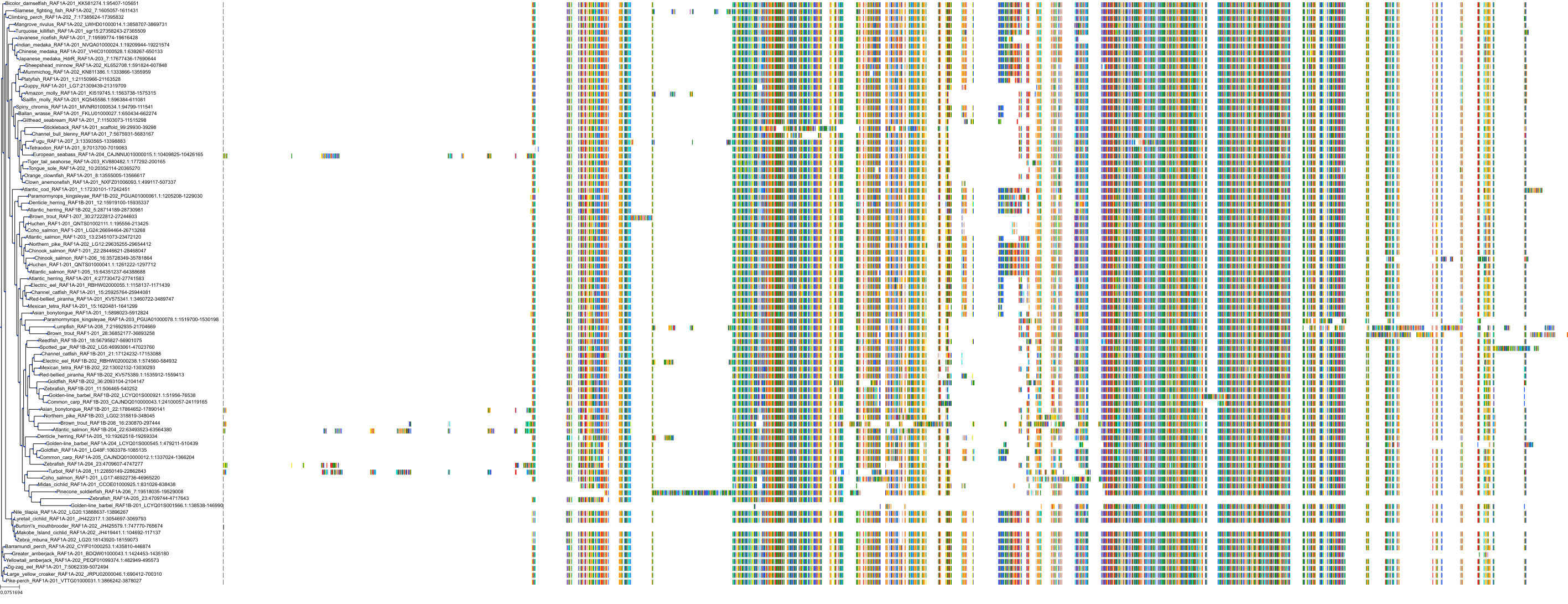
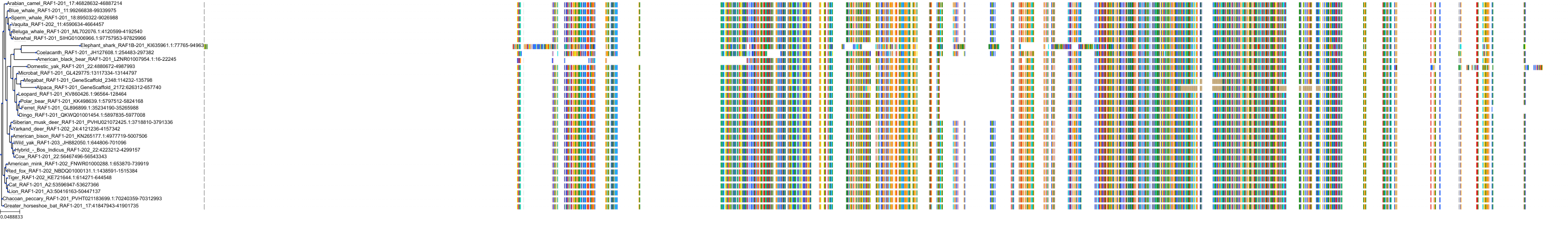
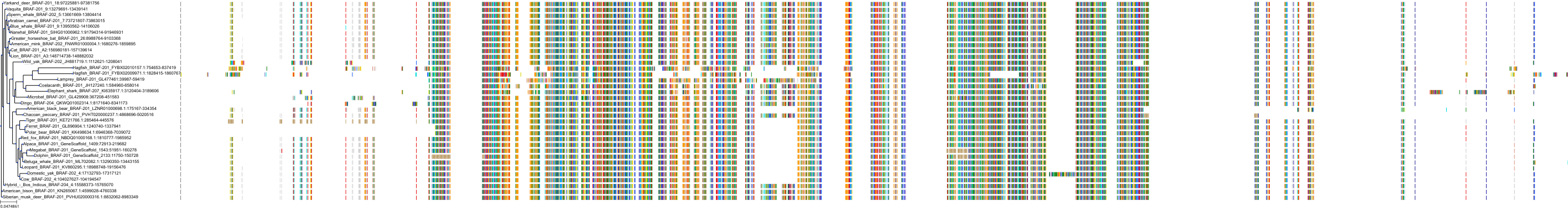
Target Conservation
|
Protein: Serine/threonine-protein kinase RAF Description: RAF proto-oncogene serine/threonine-protein kinase Organism : Homo sapiens P04049 ENSG00000132155 |
||||
|
Protein: Serine/threonine-protein kinase B-raf Description: Serine/threonine-protein kinase B-raf Organism : Homo sapiens P15056 ENSG00000157764 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL3545027 |
| DrugBank | DB12854 |
| FDA SRS | DW2NWI3TFN |
| Guide to Pharmacology | 7968 |
| SureChEMBL | SCHEMBL10049428 |
 Homo sapiens
Homo sapiens