Structure
| InChI Key | FASLTMSUPQDLIB-MHZLTWQESA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C32H36F3NO4 |
| Molecular Weight | 555.64 |
| AlogP | 7.94 |
| Hydrogen Bond Acceptor | 3.0 |
| Hydrogen Bond Donor | 2.0 |
| Number of Rotational Bond | 10.0 |
| Polar Surface Area | 75.63 |
| Molecular species | ACID |
| Aromatic Rings | 3.0 |
| Heavy Atoms | 40.0 |
Pharmacology
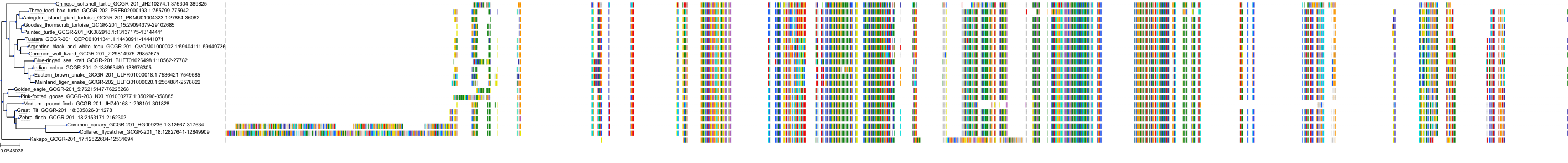
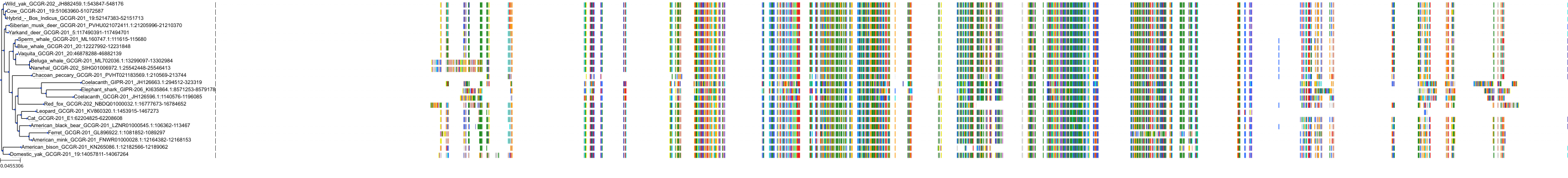
Target Conservation
|
Protein: Glucagon receptor Description: Glucagon receptor Organism : Homo sapiens P47871 ENSG00000215644 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL3707351 |
| DrugBank | DB11704 |
| FDA SRS | 74Z5ZL2KVG |
| Guide to Pharmacology | 9479 |
| PubChem | 91933867 |
| ZINC | ZINC000117040414 |