Structure
| InChI Key | FVQSSYMRZKLFDR-QRCSZXLUSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C35H42N4O6 |
| Molecular Weight | 614.74 |
| AlogP | 4.86 |
| Hydrogen Bond Acceptor | 5.0 |
| Hydrogen Bond Donor | 5.0 |
| Number of Rotational Bond | 12.0 |
| Polar Surface Area | 149.62 |
| Molecular species | ACID |
| Aromatic Rings | 3.0 |
| Heavy Atoms | 45.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Cholecystokinin B receptor antagonist | ANTAGONIST | PubMed |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Membrane receptor
Family A G protein-coupled receptor
Peptide receptor (family A GPCR)
Short peptide receptor (family A GPCR)
Cholecystokinin receptor
|
- | 0.138-30 | - | 0.1479-630.96 | - |
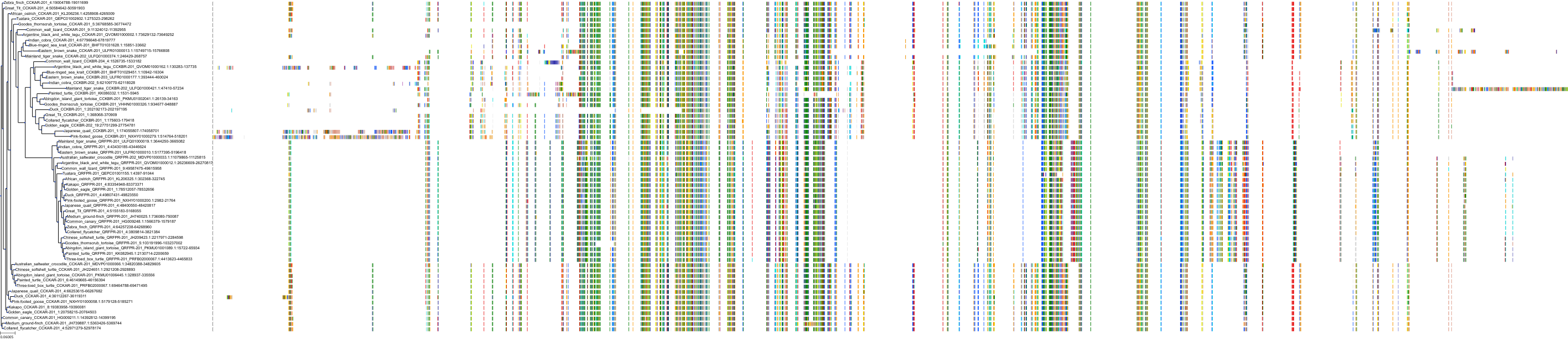
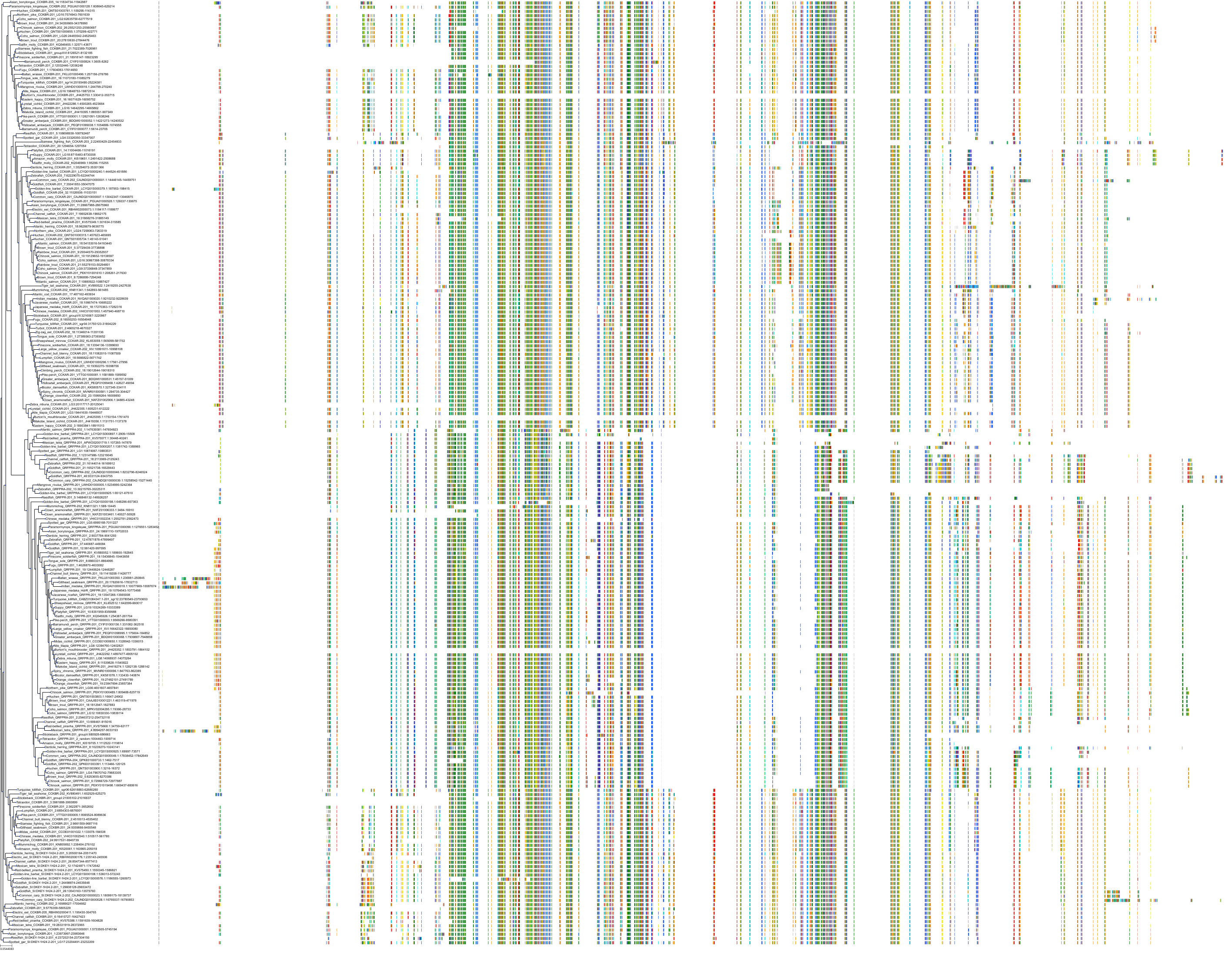
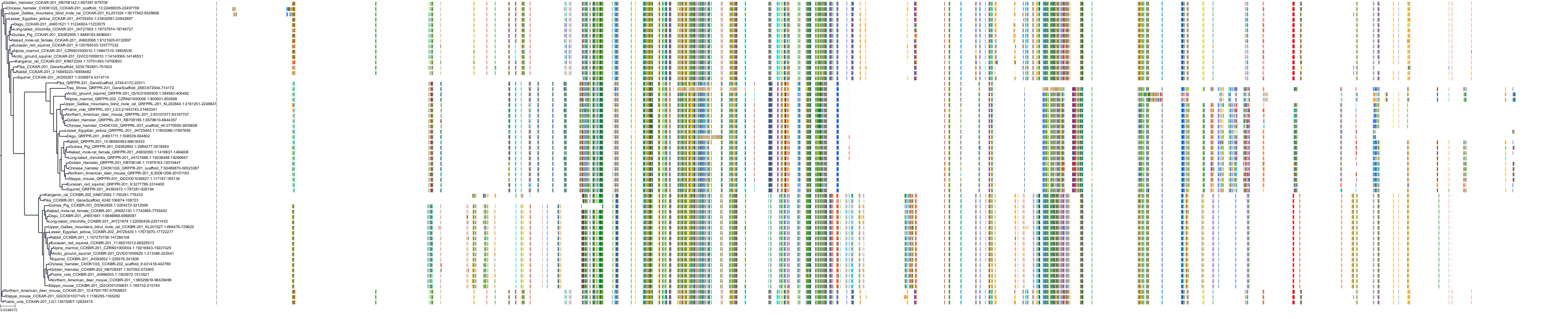
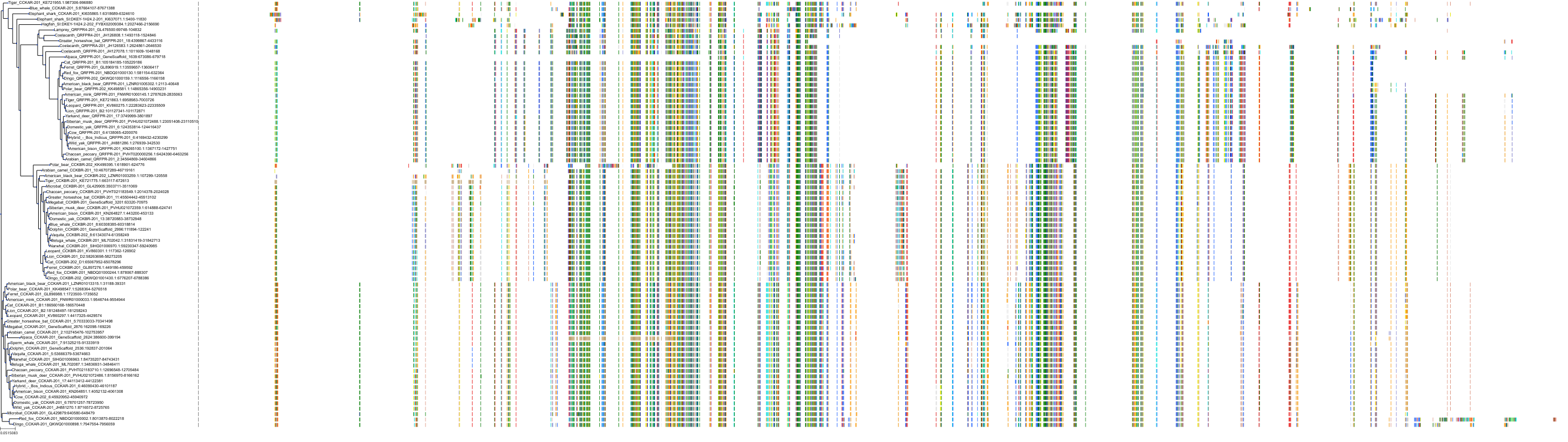
Target Conservation
|
Protein: Cholecystokinin B receptor Description: Gastrin/cholecystokinin type B receptor Organism : Homo sapiens P32239 ENSG00000110148 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL2062154 |
| FDA SRS | 2637PDX9SI |
| PubChem | 108187 |
 Cavia porcellus
Cavia porcellus
 Mus musculus
Mus musculus
 Rattus norvegicus
Rattus norvegicus