Structure
| InChI Key | CZGVOBIGEBDYTP-VSGBNLITSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C28H38N2O7S |
| Molecular Weight | 546.69 |
| AlogP | 3.91 |
| Hydrogen Bond Acceptor | 7.0 |
| Hydrogen Bond Donor | 4.0 |
| Number of Rotational Bond | 13.0 |
| Polar Surface Area | 142.03 |
| Molecular species | ACID |
| Aromatic Rings | 2.0 |
| Heavy Atoms | 38.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Ileal bile acid transporter inhibitor | INHIBITOR | PubMed |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Transporter
Electrochemical transporter
SLC superfamily of solute carriers
SLC10 family of sodium-bile acid co-transporters
|
- | 1.9-42 | - | - | - |
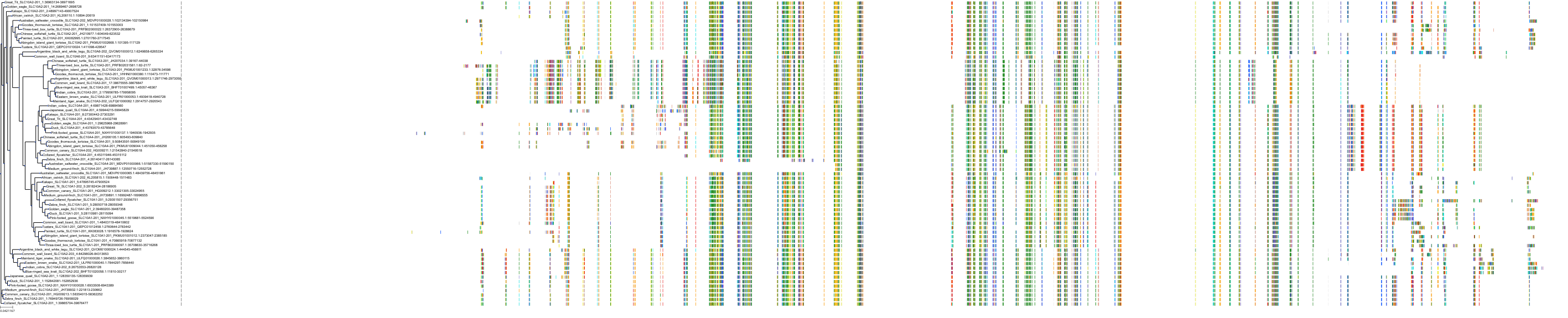
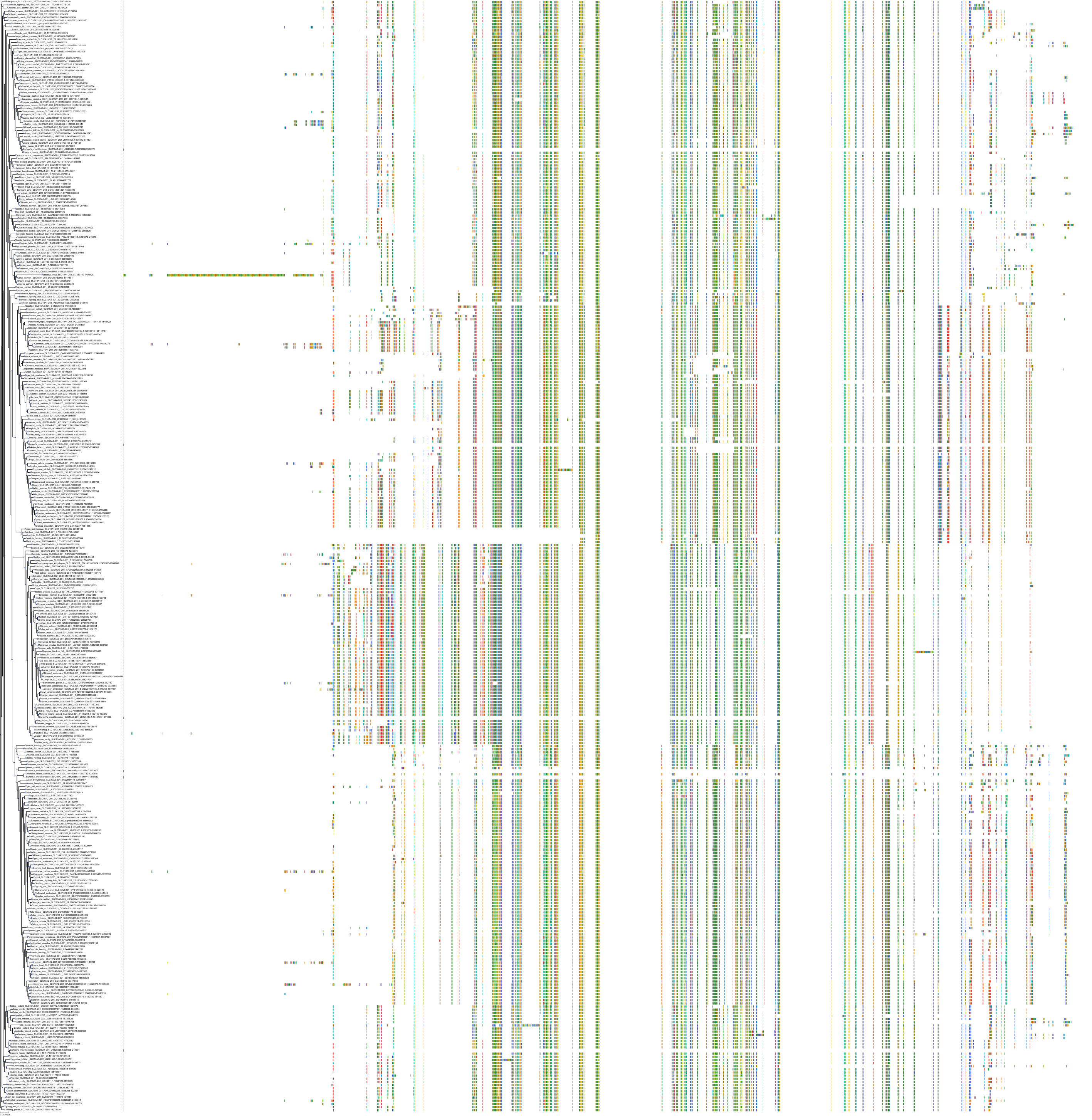
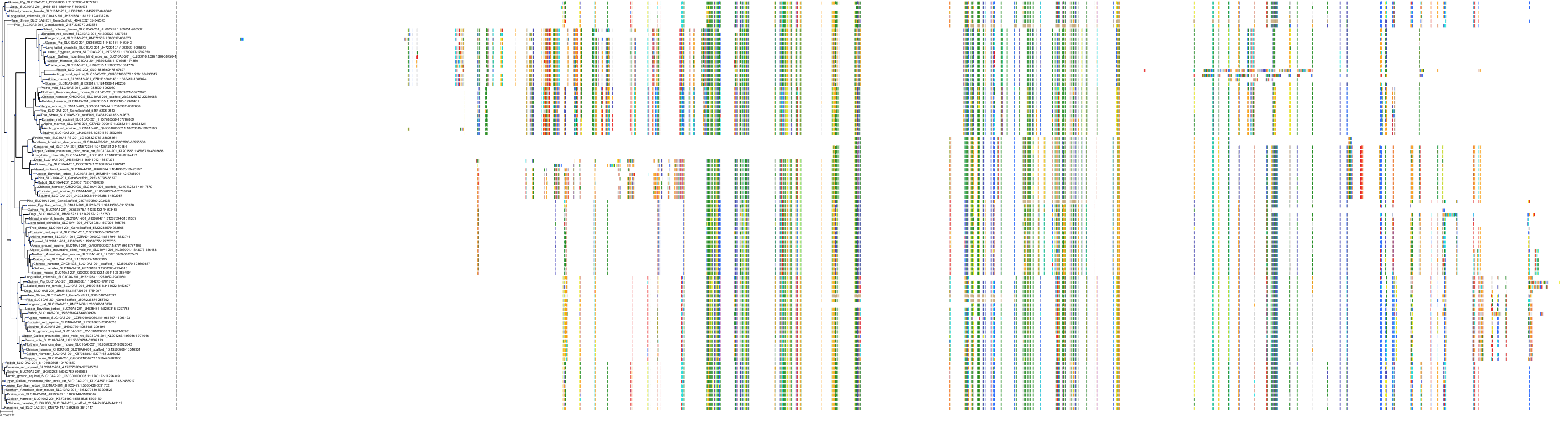
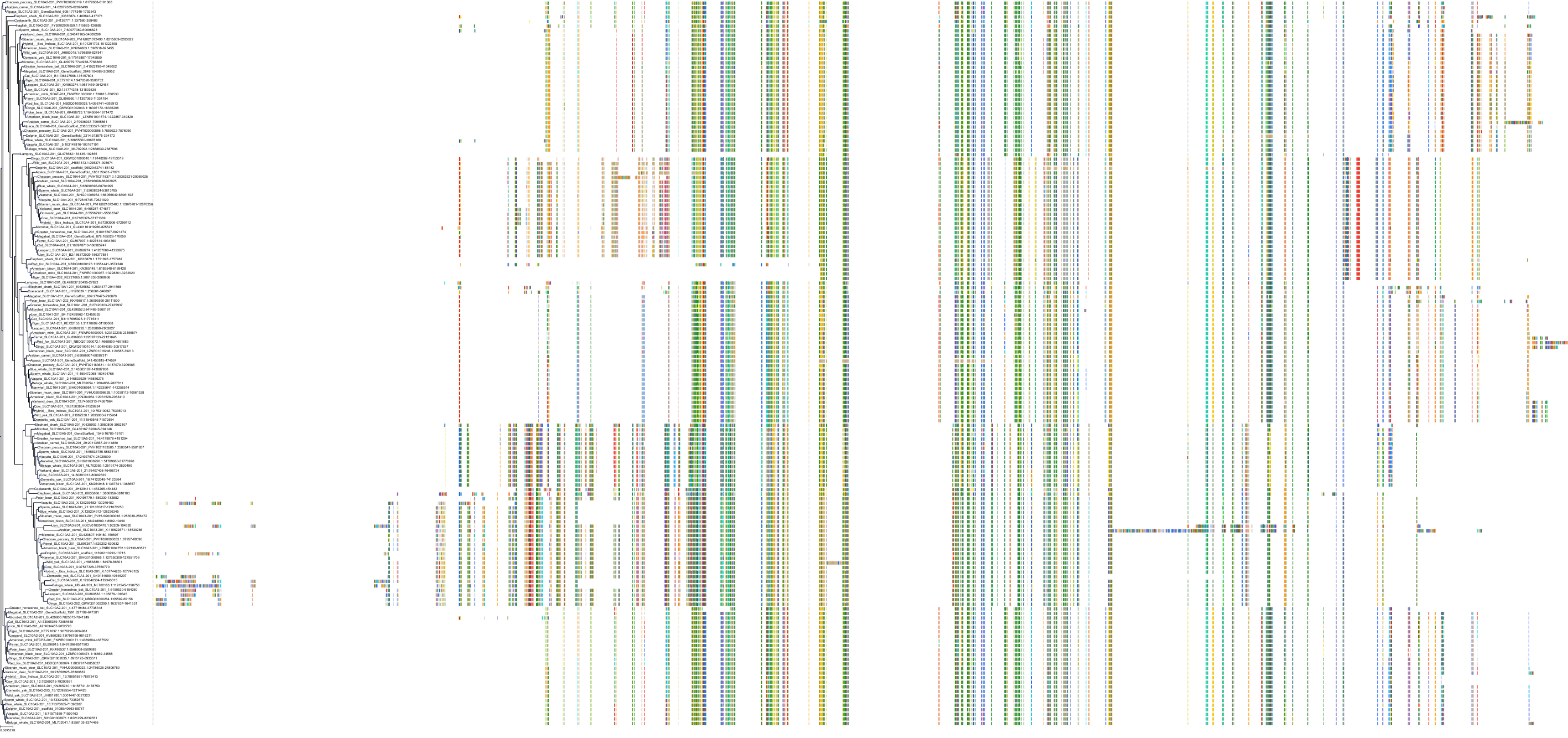
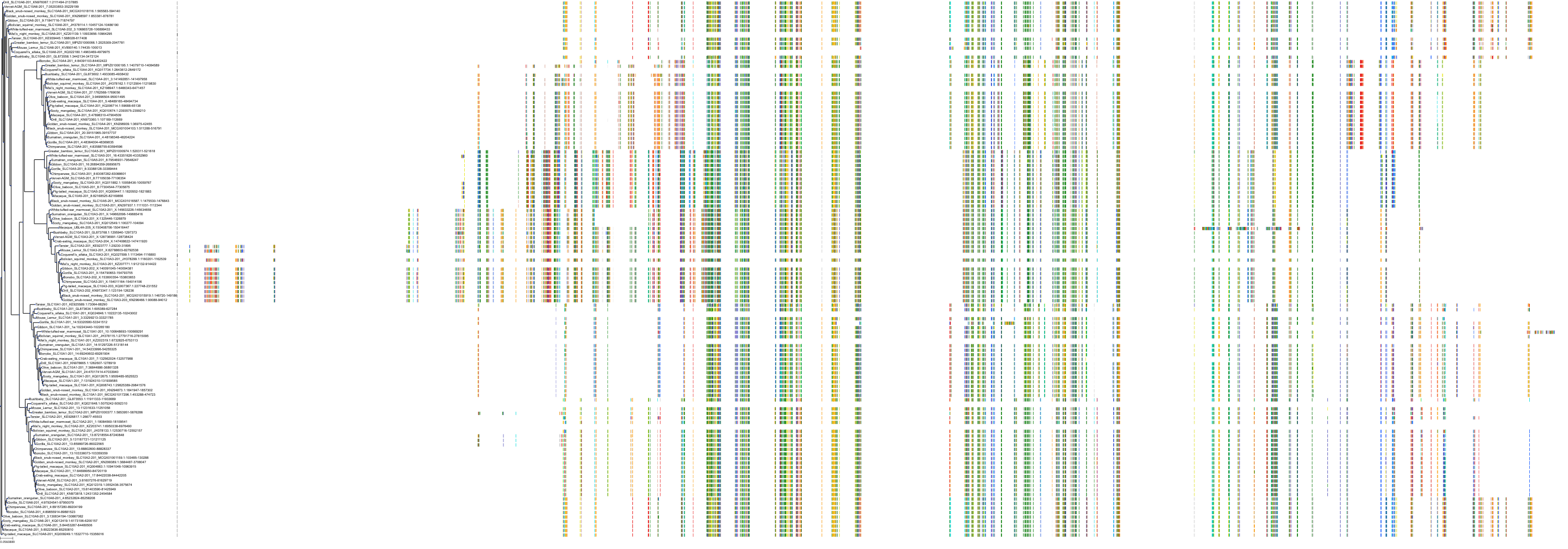
Target Conservation
|
Protein: Ileal bile acid transporter Description: Ileal sodium/bile acid cotransporter Organism : Homo sapiens Q12908 ENSG00000125255 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL2387408 |
| DrugBank | DB11729 |
| FDA SRS | 386012Z45S |
| PubChem | 53492727 |
| SureChEMBL | SCHEMBL2586025 |
| ZINC | ZINC000096270862 |
 Homo sapiens
Homo sapiens
 Mus musculus
Mus musculus
 Rattus norvegicus
Rattus norvegicus