Structure
| InChI Key | CUNDRHORZHFPLY-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C20H22N4O2 |
| Molecular Weight | 350.42 |
| AlogP | 2.9 |
| Hydrogen Bond Acceptor | 6.0 |
| Hydrogen Bond Donor | 2.0 |
| Number of Rotational Bond | 6.0 |
| Polar Surface Area | 69.87 |
| Molecular species | BASE |
| Aromatic Rings | 4.0 |
| Heavy Atoms | 26.0 |
Pharmacology
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Enzyme
Oxidoreductase
|
- | 126-148 | - | - | - |
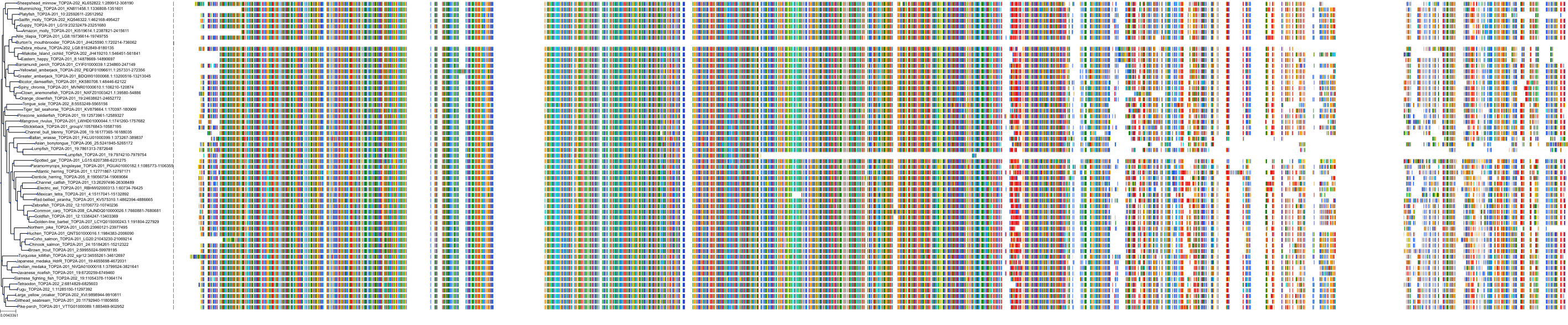
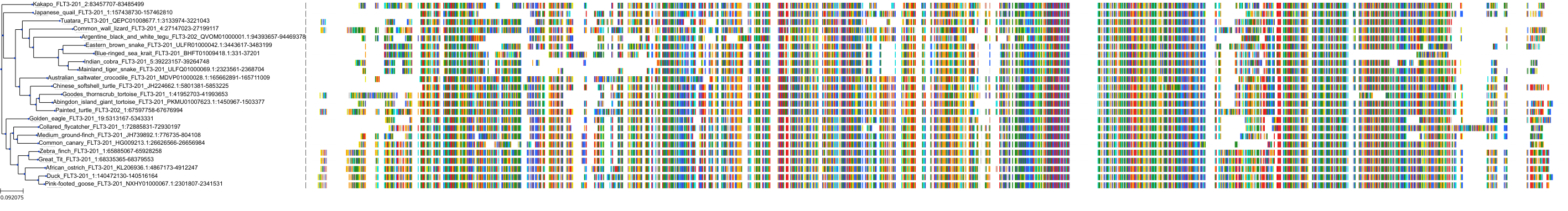
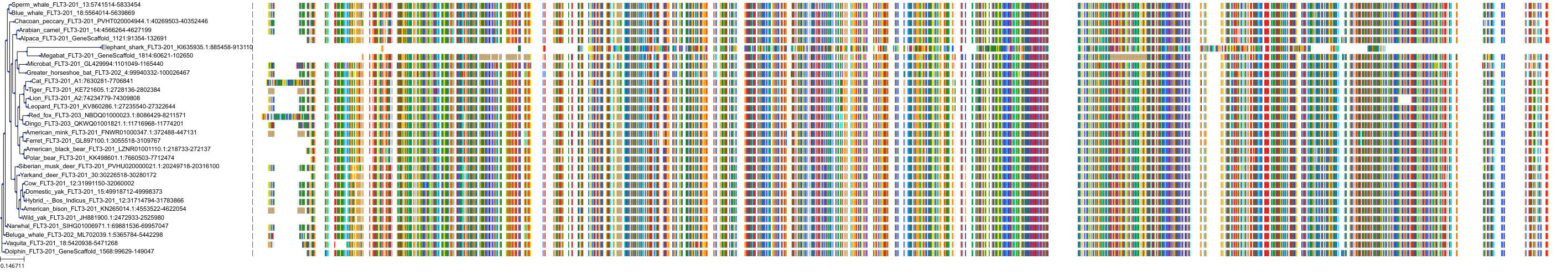
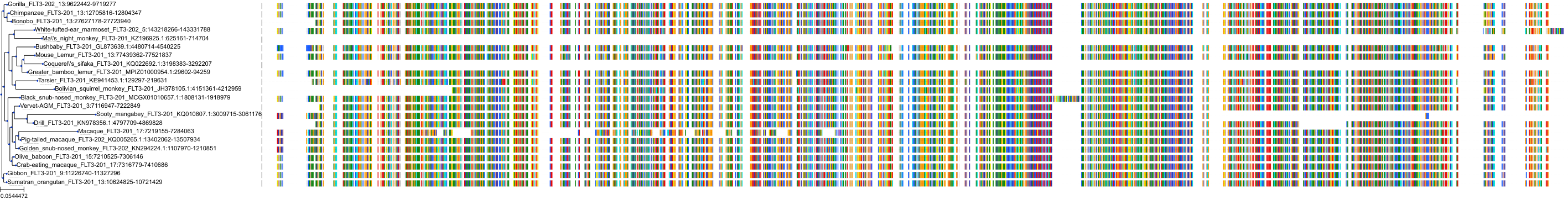
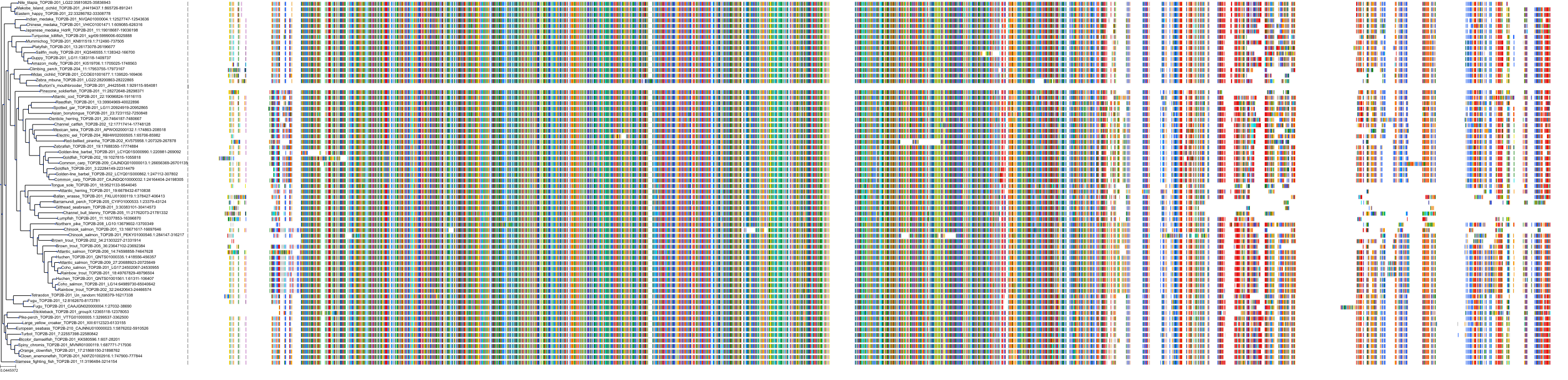
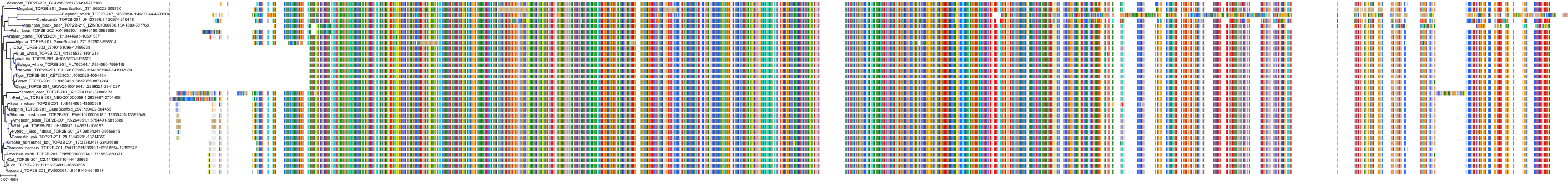
Target Conservation
|
Protein: DNA topoisomerase II Description: DNA topoisomerase 2-alpha Organism : Homo sapiens P11388 ENSG00000131747 |
||||
|
Protein: Tyrosine-protein kinase receptor FLT3 Description: Receptor-type tyrosine-protein kinase FLT3 Organism : Homo sapiens P36888 ENSG00000122025 |
||||
|
Protein: DNA topoisomerase II Description: DNA topoisomerase 2-beta Organism : Homo sapiens Q02880 ENSG00000077097 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL3545337 |
| FDA SRS | MZ4Y5H4OAB |
| PubChem | 132127 |
| ChEMBL | CHEMBL338604 |
| FDA SRS | MZ4Y5H4OAB |
| PubChem | 132127 |
| SureChEMBL | SCHEMBL2442405 |
| ZINC | ZINC000003825292 |
 Homo sapiens
Homo sapiens
 Mus musculus
Mus musculus