| Synonyms | |
| Status | |
| Molecule Category | Free-form |
| UNII | UTC046R5HM |
| EPA CompTox | DTXSID90158605 |
Structure
| InChI Key | QEMSVZNTSXPFJA-HNAYVOBHSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C20H25NO3 |
| Molecular Weight | 327.42 |
| AlogP | 2.8 |
| Hydrogen Bond Acceptor | 4.0 |
| Hydrogen Bond Donor | 3.0 |
| Number of Rotational Bond | 4.0 |
| Polar Surface Area | 63.93 |
| Molecular species | BASE |
| Aromatic Rings | 2.0 |
| Heavy Atoms | 24.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| Glutamate NMDA receptor; GRIN1/GRIN2B antagonist | ANTAGONIST | PubMed PubMed DOI |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Ion channel
Ligand-gated ion channel
Ionotropic glutamate receptor
NMDA receptor
|
- | 7-74 | - | 4.6-11 | - |
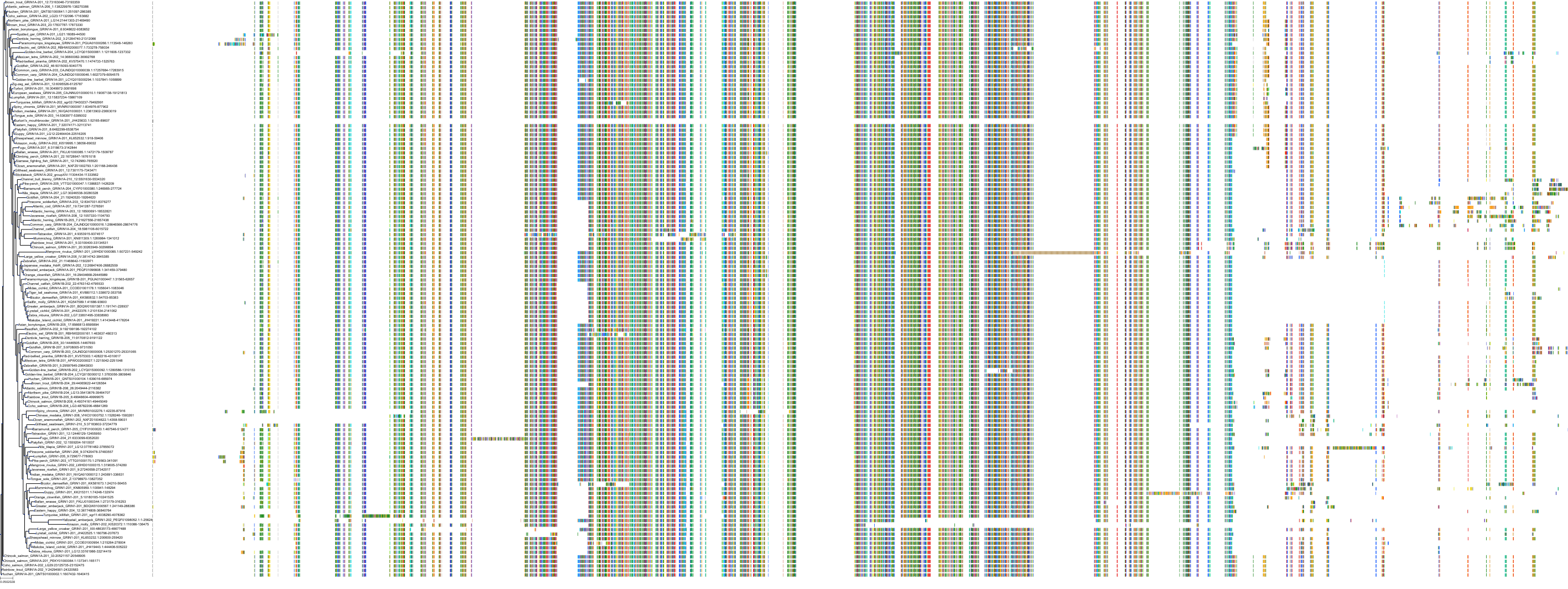
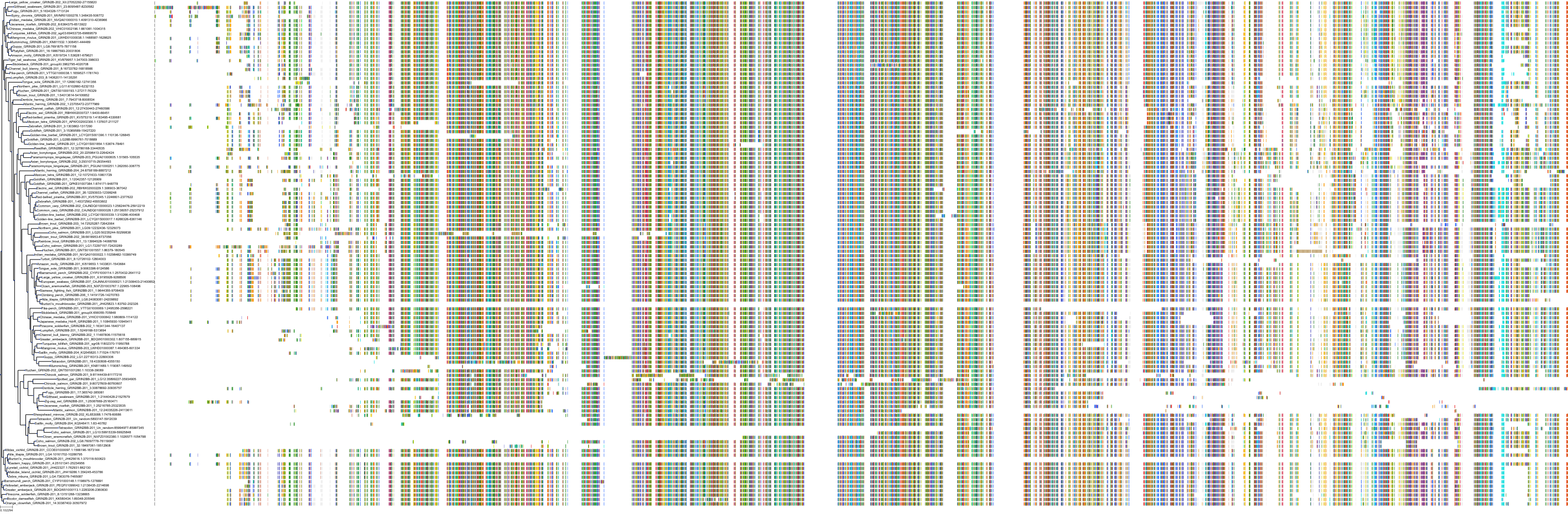
Target Conservation
|
Protein: Glutamate NMDA receptor; GRIN1/GRIN2B Description: Glutamate receptor ionotropic, NMDA 1 Organism : Homo sapiens Q05586 ENSG00000176884 |
||||
|
Protein: Glutamate NMDA receptor; GRIN1/GRIN2B Description: Glutamate receptor ionotropic, NMDA 2B Organism : Homo sapiens Q13224 ENSG00000273079 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL17350 |
| FDA SRS | UTC046R5HM |
| Guide to Pharmacology | 4163 |
| PubChem | 219101 |
| SureChEMBL | SCHEMBL4248 |
| ZINC | ZINC000000005936 |
 Homo sapiens
Homo sapiens
 Rattus norvegicus
Rattus norvegicus