Structure
| InChI Key | BBTFKAOFCSOZMB-UHFFFAOYSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C26H24N4O5 |
| Molecular Weight | 472.5 |
| AlogP | 4.39 |
| Hydrogen Bond Acceptor | 8.0 |
| Hydrogen Bond Donor | 2.0 |
| Number of Rotational Bond | 7.0 |
| Polar Surface Area | 111.67 |
| Molecular species | NEUTRAL |
| Aromatic Rings | 4.0 |
| Heavy Atoms | 35.0 |
Pharmacology
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Enzyme
Phosphodiesterase
Phosphodiesterase 4
Phosphodiesterase 4A
|
- | 2.8 | - | - | - | |
|
Enzyme
Phosphodiesterase
Phosphodiesterase 4
Phosphodiesterase 4B
|
- | 2.8 | - | - | - | |
|
Enzyme
Phosphodiesterase
Phosphodiesterase 4
Phosphodiesterase 4C
|
- | 2.8 | - | - | - | |
|
Enzyme
Phosphodiesterase
Phosphodiesterase 4
Phosphodiesterase 4D
|
- | 2.8 | - | - | - |
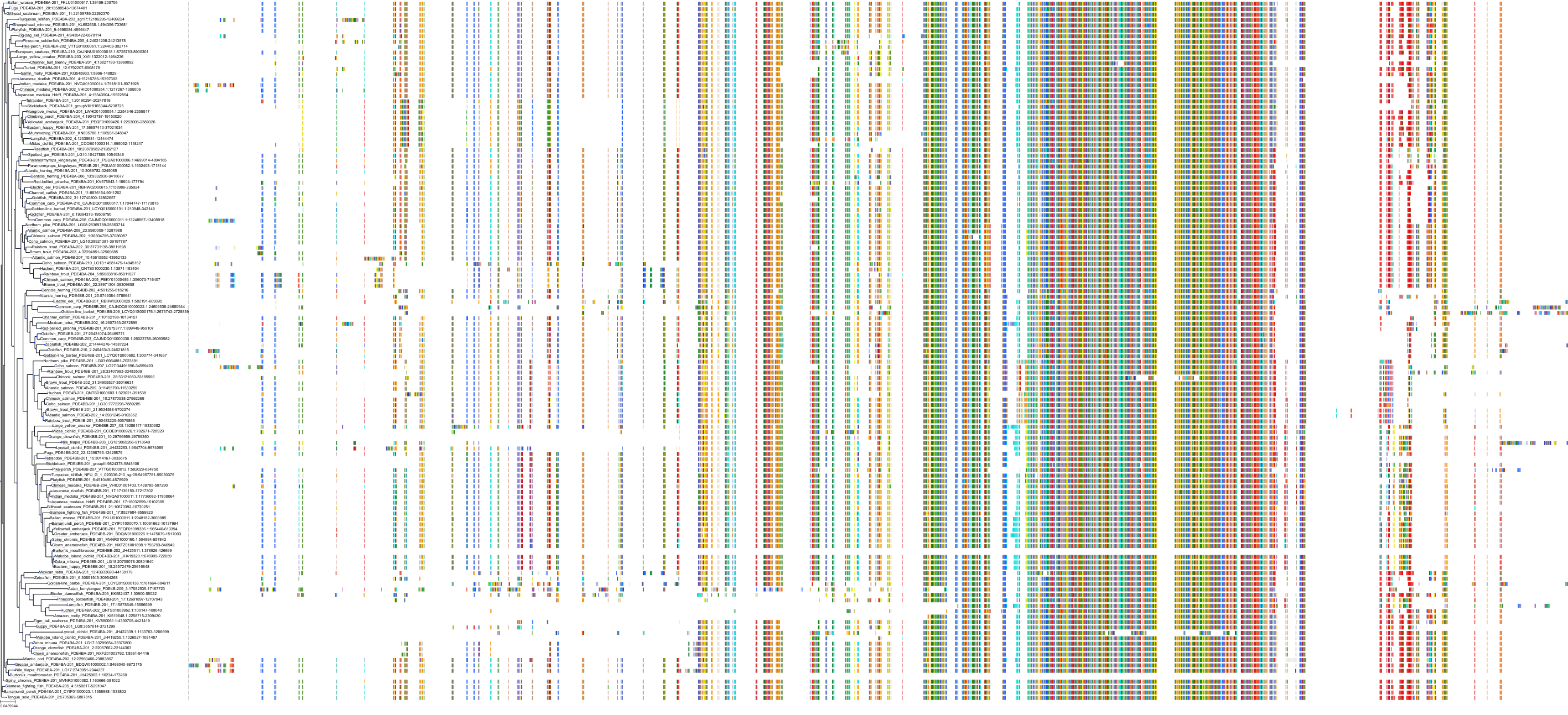
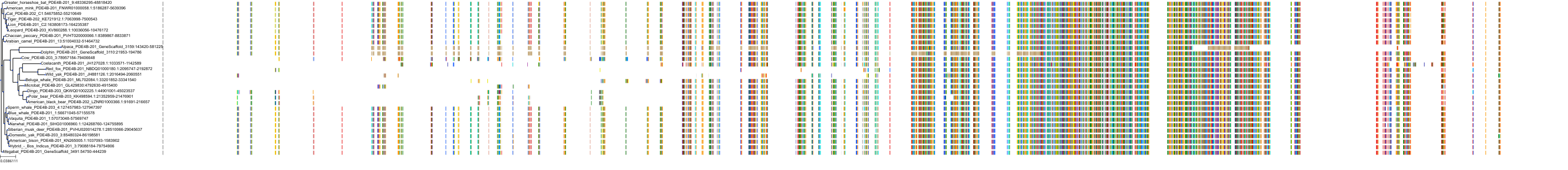
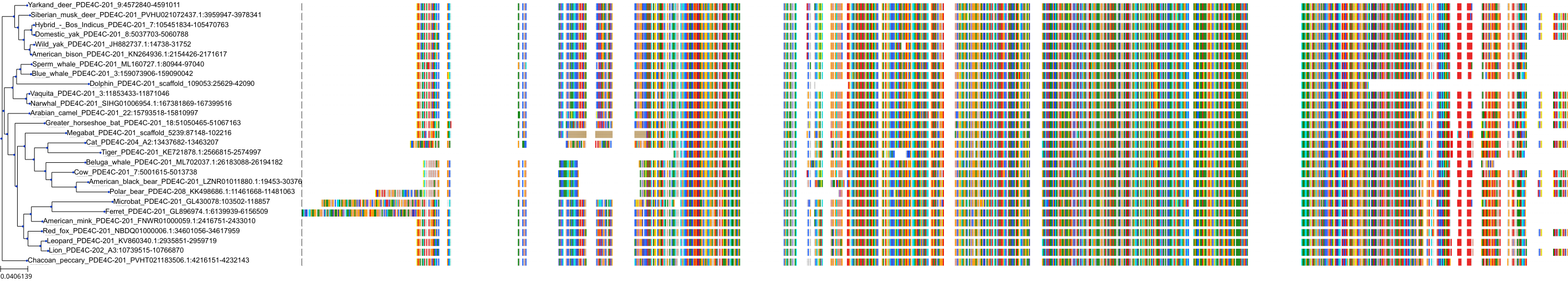
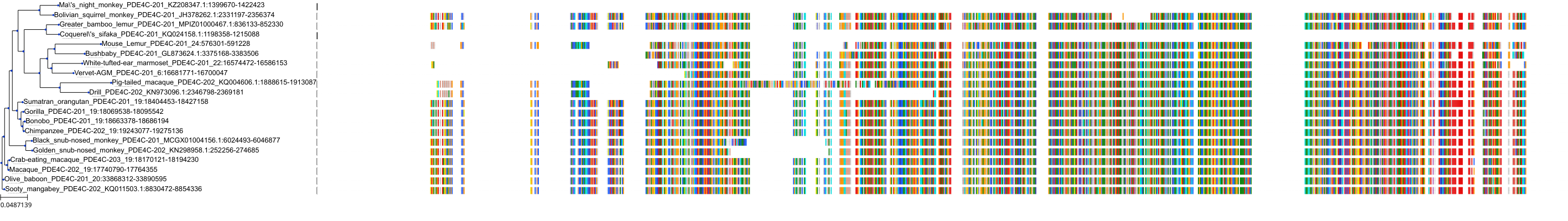
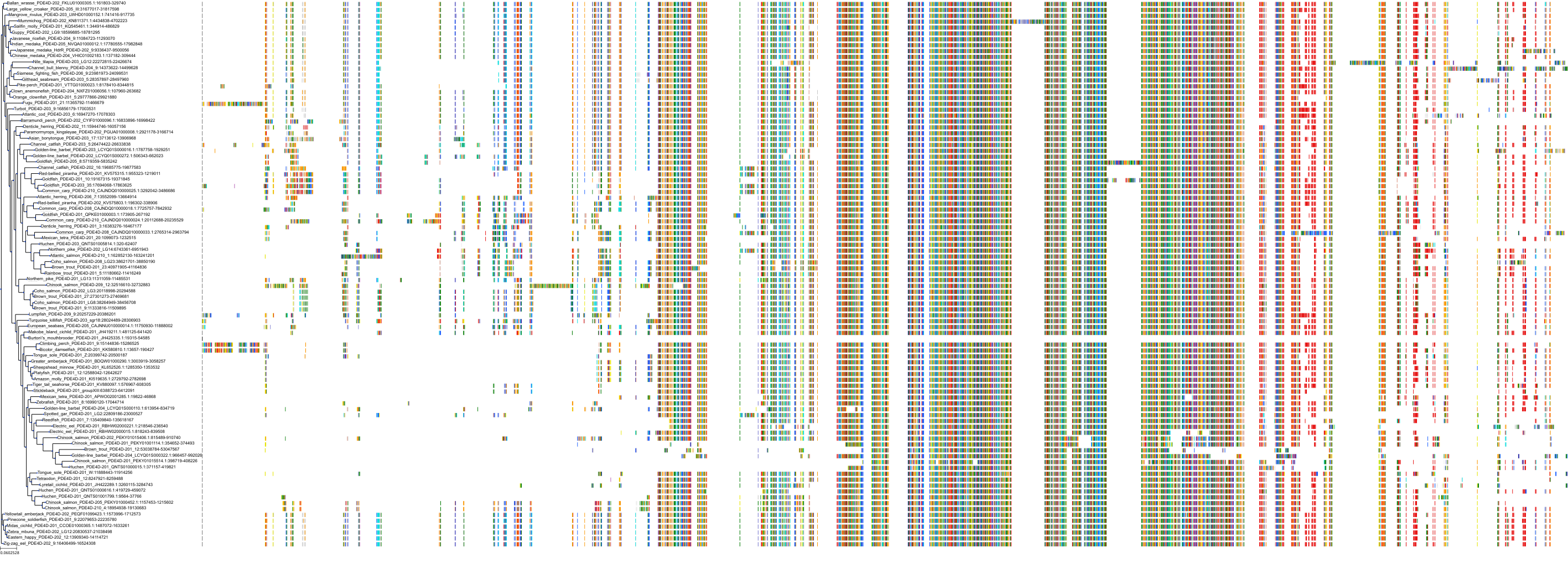
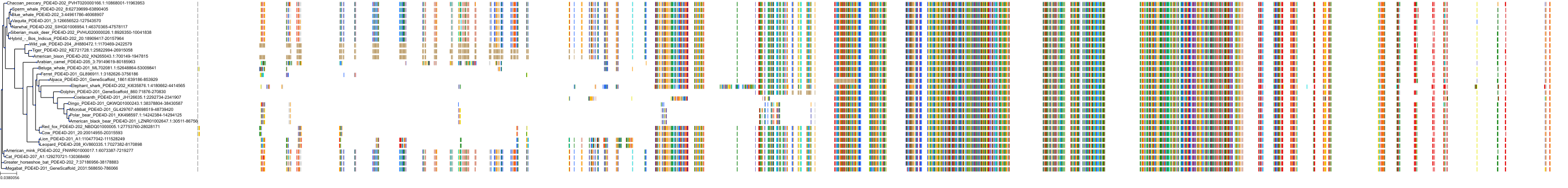
Target Conservation
|
Protein: Phosphodiesterase 4 Description: cAMP-specific 3',5'-cyclic phosphodiesterase 4A Organism : Homo sapiens P27815 ENSG00000065989 |
||||
|
Protein: Phosphodiesterase 4 Description: cAMP-specific 3',5'-cyclic phosphodiesterase 4B Organism : Homo sapiens Q07343 ENSG00000184588 |
||||
|
Protein: Phosphodiesterase 4 Description: cAMP-specific 3',5'-cyclic phosphodiesterase 4C Organism : Homo sapiens Q08493 ENSG00000105650 |
||||
|
Protein: Phosphodiesterase 4 Description: cAMP-specific 3',5'-cyclic phosphodiesterase 4D Organism : Homo sapiens Q08499 ENSG00000113448 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL3989967 |
| DrugBank | DB12776 |
| FDA SRS | TO043KKB9C |
| PubChem | 24864553 |
| SureChEMBL | SCHEMBL369445 |
| ZINC | ZINC000113676839 |
 Homo sapiens
Homo sapiens