Structure
| InChI Key | QOSMEMHKXNNIGG-SSEXGKCCSA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C31H43F2N3O5S2 |
| Molecular Weight | 639.83 |
| AlogP | 4.07 |
| Hydrogen Bond Acceptor | 6.0 |
| Number of Rotational Bond | 11.0 |
| Polar Surface Area | 95.07 |
| Molecular species | BASE |
| Aromatic Rings | 2.0 |
| Heavy Atoms | 43.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| C-C chemokine receptor type 5 antagonist | ANTAGONIST | PubMed PubMed |
| Targets | EC50(nM) | IC50(nM) | Kd(nM) | Ki(nM) | Inhibition(%) | |
|---|---|---|---|---|---|---|
|
Membrane receptor
Family A G protein-coupled receptor
Peptide receptor (family A GPCR)
Chemokine receptor
CC chemokine receptor
|
- | 0.26-0.69 | - | - | - |
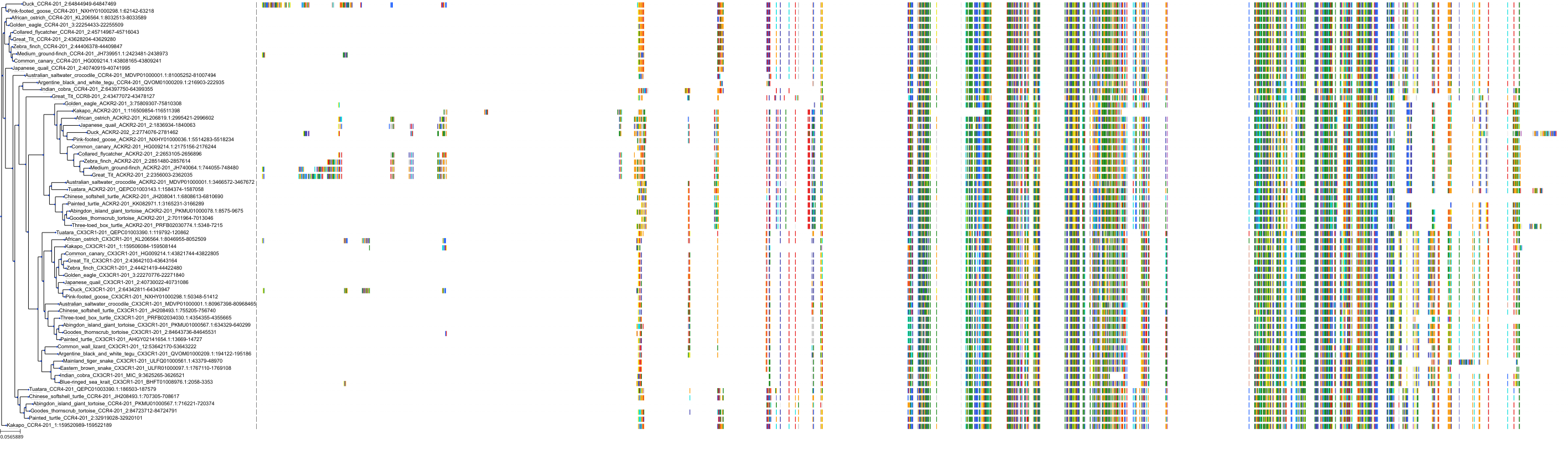
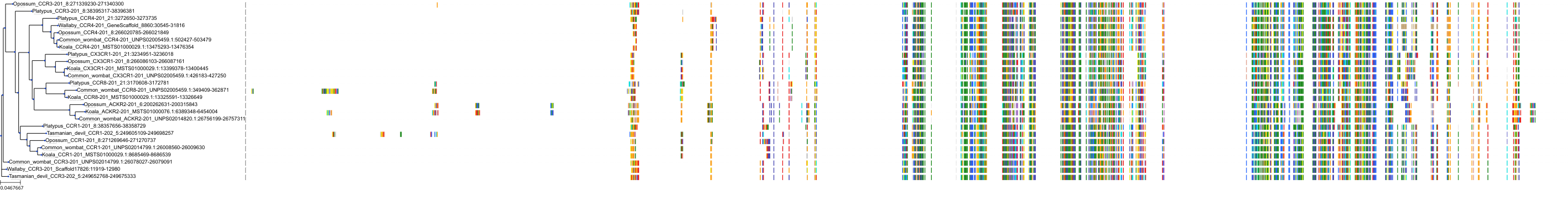
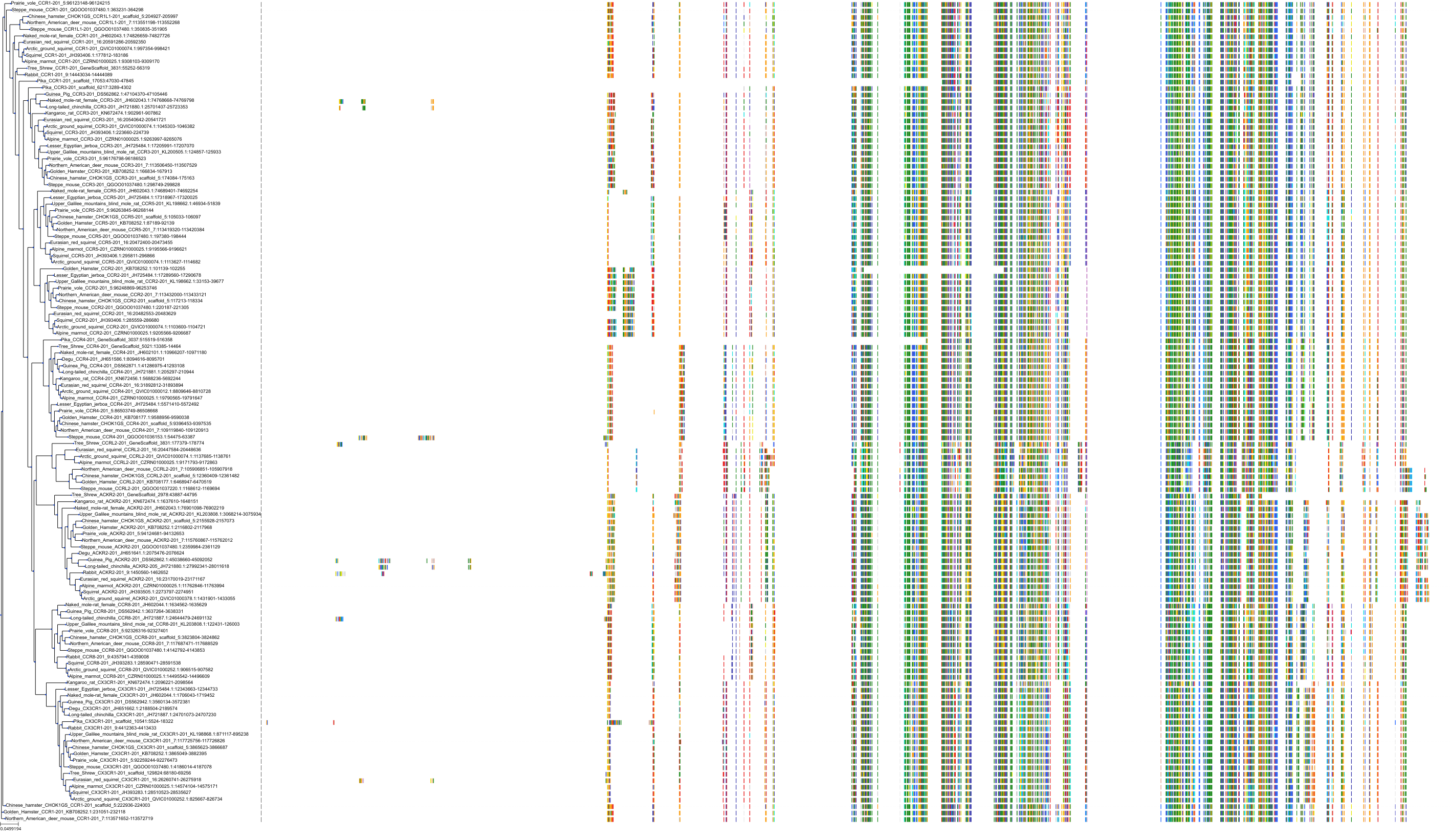
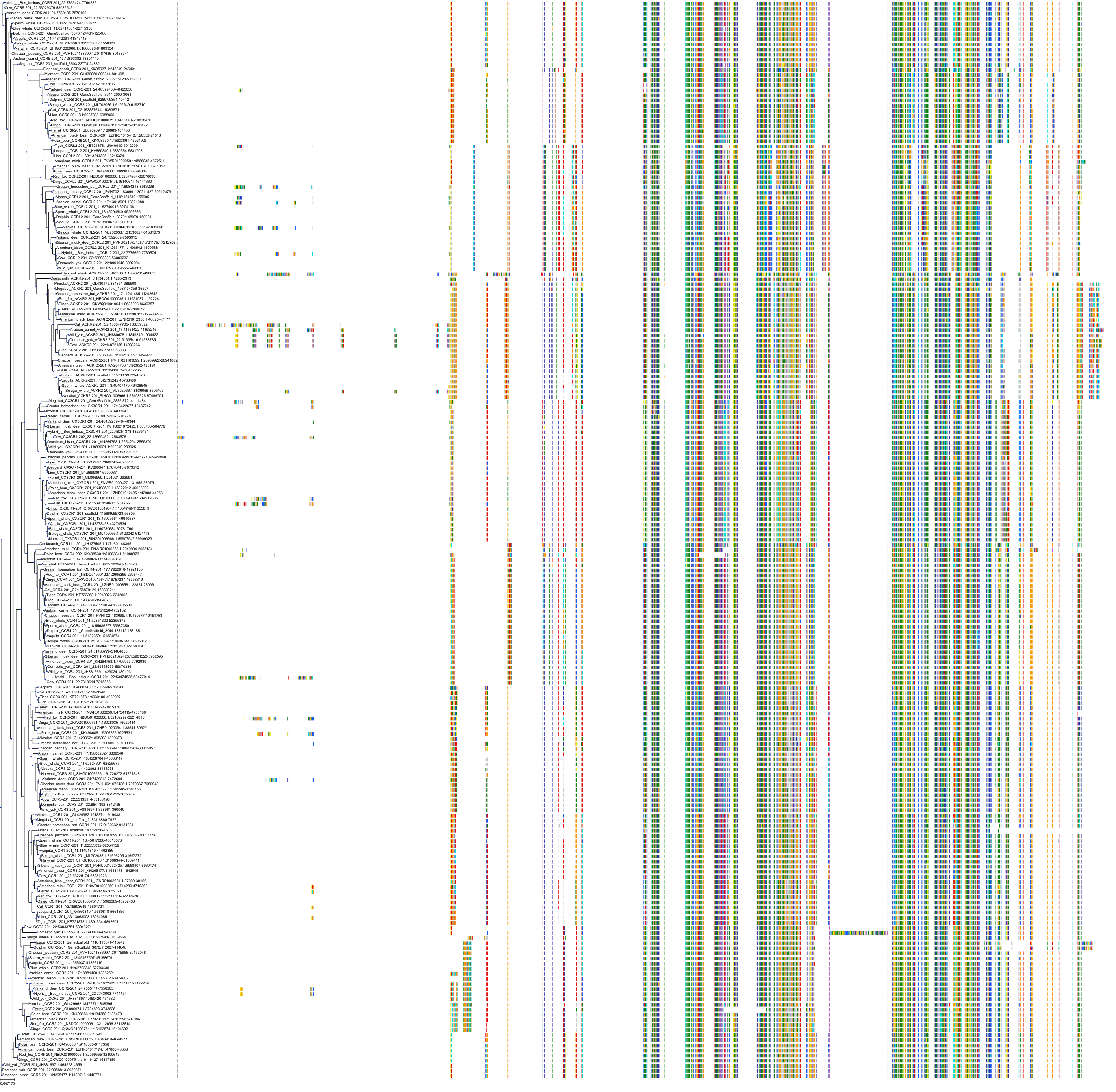
Target Conservation
|
Protein: C-C chemokine receptor type 5 Description: C-C chemokine receptor type 5 Organism : Homo sapiens P51681 ENSG00000160791 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL1951914 |
| FDA SRS | 61XQN688TW |
| Guide to Pharmacology | 7686 |
| SureChEMBL | SCHEMBL2767780 |
| ZINC | ZINC000043101474 |
 Homo sapiens
Homo sapiens