Structure
| InChI Key | XZAFZXJXZHRNAQ-STQMWFEESA-N |
|---|---|
| Smiles | |
| InChI |
|
Physicochemical Descriptors
| Property Name | Value |
|---|---|
| Molecular Formula | C18H19N5O4S |
| Molecular Weight | 401.45 |
| AlogP | 0.96 |
| Hydrogen Bond Acceptor | 9.0 |
| Hydrogen Bond Donor | 2.0 |
| Number of Rotational Bond | 5.0 |
| Polar Surface Area | 109.58 |
| Molecular species | ZWITTERION |
| Aromatic Rings | 3.0 |
| Heavy Atoms | 28.0 |
Pharmacology
| Mechanism of Action | Action | Reference |
|---|---|---|
| DNA inhibitor | INHIBITOR | PubMed |
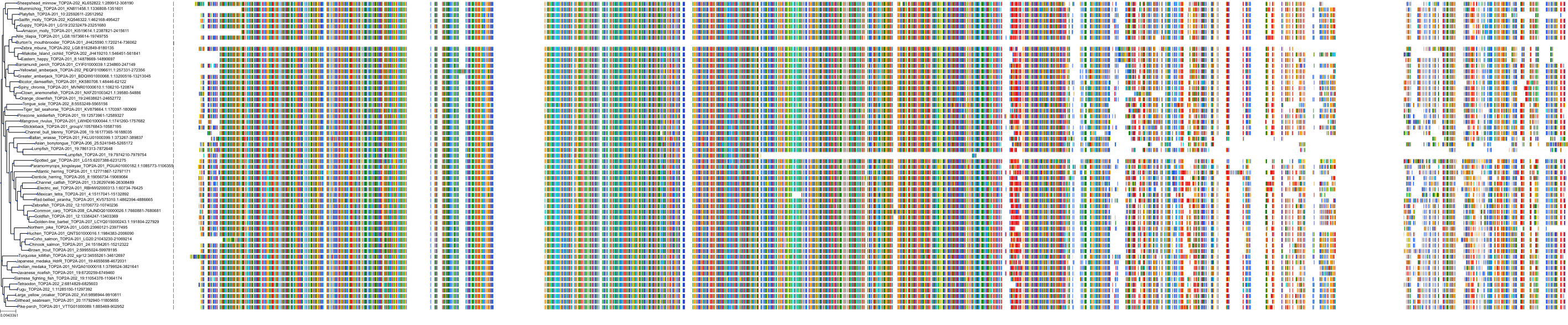
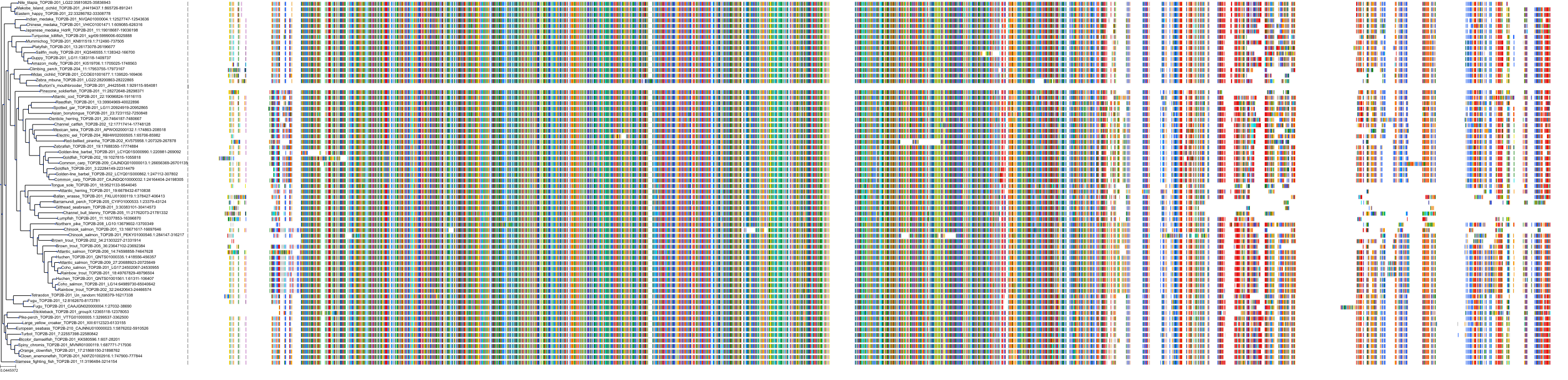
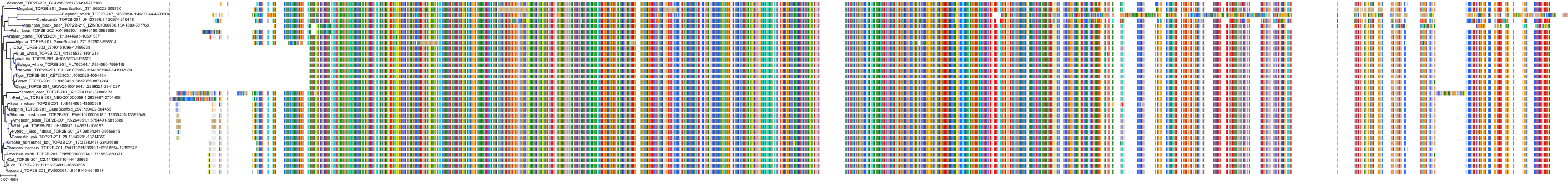
Target Conservation
|
Protein: DNA topoisomerase II Description: DNA topoisomerase 2-alpha Organism : Homo sapiens P11388 ENSG00000131747 |
||||
|
Protein: DNA topoisomerase II Description: DNA topoisomerase 2-beta Organism : Homo sapiens Q02880 ENSG00000077097 |
||||
Cross References
| Resources | Reference |
|---|---|
| ChEMBL | CHEMBL68117 |
| DrugBank | DB11999 |
| FDA SRS | K6A90IIZ19 |
| PubChem | 9952884 |
| SureChEMBL | SCHEMBL674441 |
| ZINC | ZINC000026186622 |